|
 |
| jbm > Volume 30(4); 2023 > Article |
|
Abstract
Background
Methods
Results
Conclusions
DECLARATIONS
Fig. 1
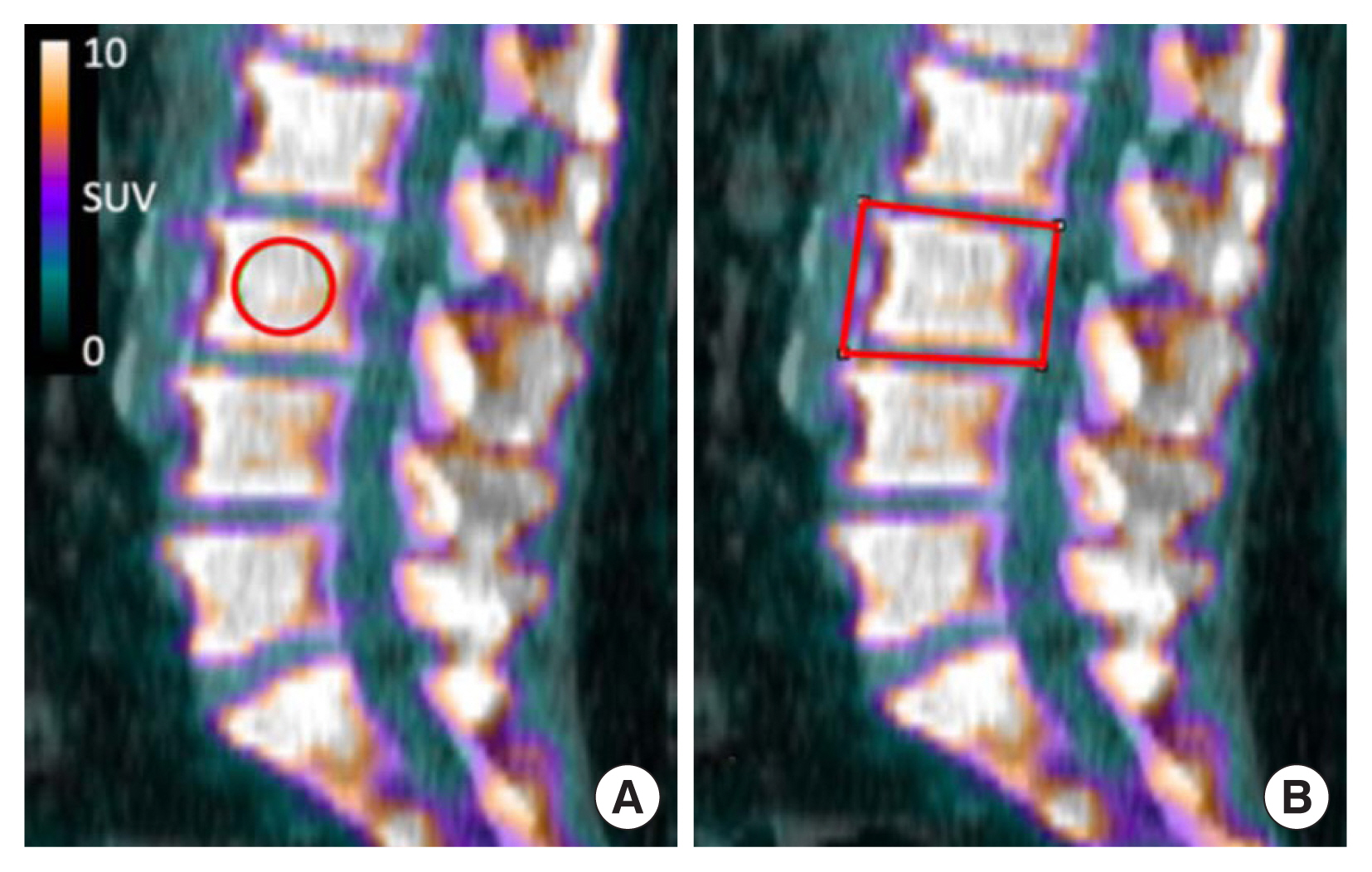
Fig. 2
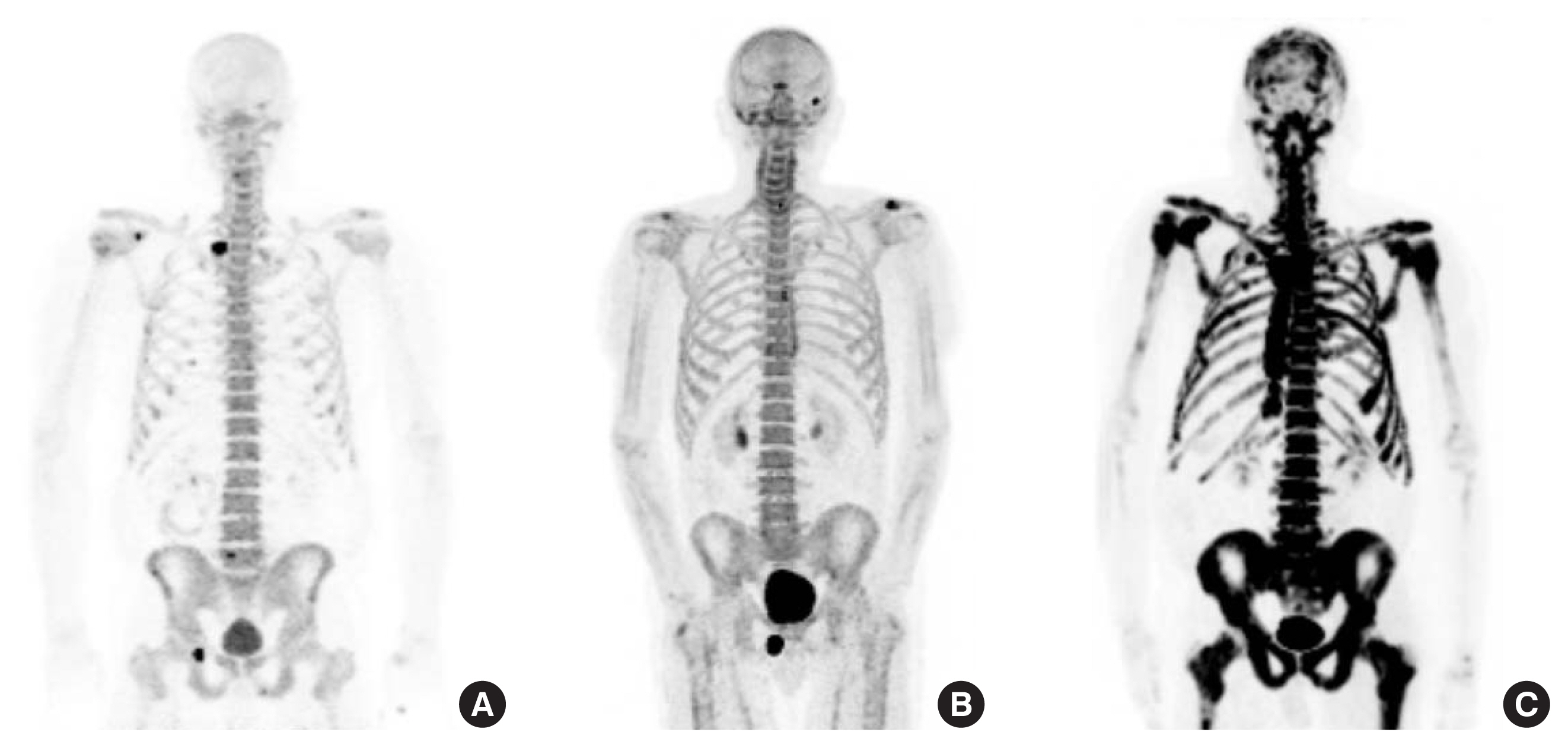
Table 1
| Variables | N | Treatment start date/radiation end date/surgery date (yr) |
|---|---|---|
| Cancer type | ||
| Metastatic | 51 | |
| Non-metastatic | 54 | |
|
|
||
| Hormone therapy | ||
| Yes | 42 | 2.1±2.8a) |
| No | 47 | |
| Unspecified | 16 | |
| Drug name (brand name) | ||
| Androgen deprivation therapy | ||
| Leuprolide (Lupron, Eligard) | 32 | |
| Degarelix (Firmagon) | 2 | |
| Anti androgens | ||
| Bicalutamide (Casodex) | 23 | |
| Nilutamide (Nilandron) | 1 | |
| Enzalutamide (Xtandi) | 4 | |
| Triple androgen blockade | ||
| Dutasteride (Avodart) | 2 | |
| Bone health | ||
| Denosumab (Xgeva) | 3 | |
|
|
||
| Radiation therapy | ||
| Yes | 45 | 6.9±5.1b) |
| No | 48 | |
| Unspecified | 12 | |
|
|
||
| Radiation type | ||
| External beam radiation therapy | 12 | |
| Brachytherapy | 6 | |
| Intensity-modulated radiation therapy | 6 | |
| Salvage radiation therapy | 6 | |
| Unspecified | 15 | |
|
|
||
| Radical prostatectomy | ||
| Yes | 43 | 7.0±6.3b) |
| No | 52 | |
| Unspecified | 10 | |
Table 2
| Fracture location | Na) | Time to fracture (yr) | Description |
|---|---|---|---|
| Spine | 14 | 2.9 | T2, T3, T4, T5, T7 (3), midthoracic, T8, T9, T10, T11, T12, L1, L2 (2), L3 (2), L1-4 transverse processes, L2-L3, hemisacrum, unspecified |
| Rib | 8 | 2.1 | |
| Hip | 6 | 4.2 | |
| Arm/Leg/Foot | 6 | 4.0 |
Table 3
| Fracture (N=27) | No fracture (N=78) | P-value | Dose-corrected P-value | |
|---|---|---|---|---|
| Thoracic SUVmean (R) | 5.2±0.7 | 5.7±1.3 | 0.0192 | 0.0429 |
| Thoracic SUVmean (S) | 6.6±1.2 | 7.3±1.7 | 0.0215 | 0.0420 |
| Thoracic SUVmax (R) | 8.6±2.1 | 9.9±2.8 | 0.0136 | 0.0204 |
| Thoracic SUVmax (S) | 7.9±1.7 | 8.8±2.2 | 0.0364 | 0.0563 |
| Thoracic SUVpeak (R) | 7.3±1.4 | 8.3±2.1 | 0.0083 | 0.0163 |
| Thoracic SUVqpeak (R) | 7.9±1.8 | 9.1±2.4 | 0.0111 | 0.0170 |
| Thoracic HU (R) | 168.6±33.7 | 175.9±39.7 | 0.3708 | - |
| Agea) | 71.7±8.1 | 70.5±7.9 | 0.4944 | - |
| BMIa) | 29.8±5.3 | 29.0±5.1 | 0.5372 | - |
| Minutes to scana) | 68.1±12.7 | 69.5±14.3 | 0.6522 | - |
| Radiotracer dosea) | 8.2±2.7 | 7.9±2.4 | 0.5345 | - |
a) Age, BMI, time to scan, and tracer dosage were not significantly different between the fracture and no-fracture groups.
SUVmean, mean standardized uptake value; R, rectangular region of interest; S, spherical region of interest; SUVmax, maximum standardized uptake value; SUVpeak, peak standardized uptake value; SUVqpeak, the average of SUVmax together with the 3 hottest pixels in a 1 cc volume, centered on SUVmax; HU, Hounsfield units; BMI, body mass index.
Table 4
| Compression fracture (N=14) | No compression fracture (N=91) | P-value | |
|---|---|---|---|
| Cervical HU (R) | 234.1±45.7 | 283.6±73.9 | 0.0030 |
| Thoracic HU (R) | 160.2±28.9 | 176.0±39.2 | 0.0958 |
| Lumbar HU (R) | 148.4±30.6 | 151.3±37.5 | 0.7673 |
| Sacral HU (R) | 146.7±27.4 | 166.4±45.0 | 0.0456 |
| Average spine HU | 175.9±19.2 | 198.7±43.2 | 0.0003 |
| Agea) | 73.4±8.4 | 70.4±7.8 | 0.2213 |
| BMIa) | 29.5±6.2 | 29.2±5.0 | 0.8408 |
| Minutes to scana) | 71.1±15.9 | 68.9±13.6 | 0.6558 |
| Radiotracer dosea) | 7.9±2.8 | 7.9±2.5 | 0.9900 |
Table 5
REFERENCES
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,437 View
- 40 Download
- ORCID iDs
-
Helene Chesnais

https://orcid.org/0000-0002-3936-726XNikita Bastin

https://orcid.org/0000-0002-6058-2634Sofia Miguez

https://orcid.org/0000-0003-3912-1996Daniel Kargilis

https://orcid.org/0000-0001-7013-4301Anita Kalluri

https://orcid.org/0000-0002-5783-3877Ashley Terry

https://orcid.org/0000-0001-9800-6598Chamith S. Rajapakse

https://orcid.org/0000-0002-9247-833X - Related articles





 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print