|
 |
| jbm > Volume 30(3); 2023 > Article |
|
Abstract
Vitamin D (VD) exerts a wide variety of biological actions in addition to its well-known roles in calcium homeostasis. Nutritional VD deficiency induces rachitic abnormalities in growing children and osteomalacia in adults, and it has been proposed to underlie the onset and development of multiple non-communicable chronic diseases. Therefore, the administration of VD or synthetic VD analogues represents a promising therapeutic strategy; indeed, VD and a VD agonist have shown clinical promise in mitigating osteoporosis and symptoms of insufficient calcium intake. However, even though high doses of VD analogues have shown pre-clinical efficacy against several diseases, including cancers, they have not yet had wide-spread clinical success. This difference may be due to limitation of clinical doses in light of the inherent calcemic action of VD. An approach to overcome this problem involves the development of VD analogues with lower calcemic activity, which could be administered in high doses to attenuate the onset and progress of disease. In a similar strategy, selective estrogen receptor modulators have had success as anti-osteoporosis drugs, and they have shown benefit for other estrogen target organs by serving as partial antagonists or agonists of estrogen receptor α. It is thus conceivable to generate synthetic partial antagonists or agonists for the VD receptor (VDR) that would exert beneficial effects on bone and other VD target organs. In this review, we discuss the molecular basis of the development of such synthetic VDR ligands from the viewpoint of roles of VDR in gene regulation.
Vitamin D (VD) is a fat-soluble vitamin, and it serves as one of the major hormones supporting bone health and calcium homeostasis.[1-4] Nutritional VD deficiencies can cause rickets in higher mammals during growth stages, and it can lead to osteomalacia in adults.[5] In addition, hereditary rickets can be caused by the genetic impairment of VD processing pathways or related signaling pathways. [6,7]
While genetic impairments of VD-associated proteins often lead to some generally similar outcomes, subtle differences in the symptoms caused by different genetic alterations can provide insight into the biological aspects of VD. For example, 2 types of hereditary rickets both cause bone disorders, but they differentially impact the skin. Type 1 hereditary rickets is caused by decreased activity of cytochrome P450 (CYP) 27B1, a key enzyme of VD biosynthesis that converts a VD precursor (25-hydroxy-VD3 [25(OH)D3]) into an active form of VD, 1α,25-dihydroxy-VD3 (1α,25[OH]2D3). [6] Type 2 hereditary rickets is caused by a malfunction of the VD receptor (VDR). Patients with type 2 hereditary rickets exhibit an identical rachitic abnormality to those with type 1 hereditary rickets, but type 2 is also characterized by alopecia, which is undetectable in type 1 patients (Fig. 1). These forms of hereditary rickets have been experimentally recapitulated by introducing null mutations into the mouse genes encoding CYP27B1 and VDR, and alopecia is observed only in mice that are genetically deficient in VDR. [4,6] This difference in outcomes between subjects lacking active VD and those lacking active VDR provides further evidence of the complexity of VD signaling and that it has tissue-specific effects.
In addition to its involvement in calcium metabolism, a wide variety of other biological events, including inflammation and cell proliferation, appear to be facilitated by VD.[7,8] Moreover, the dietary intake of sufficient VD is considered to slow the onset and development of many non-communicable chronic diseases, and multiple epidemiological studies have demonstrated correlations of the risks of particular disease events with nutritive VD deficiencies.[9,10] Unfortunately, dietary VD deficiencies are relatively common worldwide, even in some people with optimal diets.[2,11] As many disorders, including cancer, [12,13] have been related to inflammatory events, VD has been assumed to attenuate the inflammation process, though that hypothesis remains confirmed.[14-17] Given the fact that dietary VD deficiency is quite common globally, particularly in older adults, [2,11] an adequate intake of VD from diet and supplementation has been recommended to improve bone health and overall quality of life. [7,8] Additionally, VD and related pathways have been targeted in multiple therapeutic strategies.
As VD deficiency is considered to be a risk factor for the development of cancer, multiple clinical trials of therapies involving 1α,25(OH)2D3 or synthetic VD analogues have been conducted in the context of a variety of cancer types.[18,19] While treatments with 1α,25(OH)2D3 and synthetic VD analogues attenuate the proliferation of cultured cancer cell lines and the growth of xenografted tumors derived from certain types of human cancer cell lines, they have not yet been proven to inhibit the onset or development of cancers in human patients.[18-20]
The occurrence of several other non-communicable chronic diseases, including type 2 diabetes and hypertension, has been connected in some studies to the nutritional status of VD, though epidemiological outcomes have not been consistent among the reports.[9,10] However, studies of other disorders have suggested that VD alleviates inflammation in the context of multiple diseases and pathological events.[14,15] Despite these connections, similar to the clinical failure of VD administration to cancer patients, the clinical effects of VD supplementation and its analogues in other diseases remain to be established.
The lack of effectiveness of VD in clinical applications might be due in part to the relatively low doses of VD and synthetic analogues that can be administered.[21,22] Because these compounds are calcemic, over-supplementation can lead to hypercalcemia, and doses that are sufficiently high to attenuate disease development are thus not clinically applicable. Though the calcemic activities of VDR ligands are indispensable for bone health, the clinical potential of VDR ligands that specifically exert anti-inflammatory action would be desirable, and the development of such a new generation of VDR ligands is eagerly anticipated.
VD is derived from dietary sources as a fat-soluble vitamin, but it is also produced endogenously from VD precursors.[6,23] The active form of VD, 1α,25(OH)2D3, is generated from its precursor (VD3) through 2 steps of hydroxylation, which are mainly catalyzed by hepatic CYP2R1 and renal CYP27B1. VD3 is first hydroxylated by CYP2R1 to form 25(OH)D3, which is subsequently converted into 1α,25(OH)2D3 by CYP27B1. Since CYP27B1 expression in the renal proximal tubule is highly sensitive and reciprocally regulated by parathyroid hormone (positive) and 1α,25(OH)2D3 (negative), the production of 1α,25(OH)2D3 is normally optimally balanced to meet bodily demands for serum VD.[6,23] When serum 1α,25(OH)2D3 is in excess, the activity of CYP27B1 is reduced, and 25(OH)D3 is hydroxylated by CYP24A1 in the proximal tubules to form 24,25(OH)2D3, which cannot be directly converted to the active form.[5,6]
Reflecting the significance of CYP27B1 and CYP24A1 in the tight regulation of the serum levels of 1α,25(OH)2D3, genetic inactivation of the gene encoding CYP27B1 causes type 1 hereditary rickets. In addition, inactivating genetic mutations of the gene encoding CYP24A1 were initially assumed to underlie the clinical symptoms of idiopathic infantile hypercalcemia.[24,25] Upon genomic sequencing of 5 patients, 5 genetic mutations were indeed identified in regions of the CYP24A1 gene that are important for the catalytic activity of the enzyme, and all 5 of the CYP24A1 mutant proteins were shown to be impaired in hydroxylation of 25(OH)D3. More recently, similar genetic mutations in CYP24A1 were found in adults, and these cases have suggested that inactivating mutations of this gene lead to hypercalcemia.[26] Mild inactivation of the CYP24A1 gene by single nucleotide polymorphisms or other genetic mutations has been discussed as a risk factor for the development of hypercalcemia in certain pathological settings.
VDR, a member of the nuclear steroid hormone receptor superfamily, mediates the intracellular signaling of VD. Nuclear receptors (NRs), including VDR, act as ligand-dependent transcription factors that interact with response elements in the promoters of target genes, which include protein-coding genes as well as genes that code for non-translated RNA.[26,27] In the absence of VD, VDR is localized in the cytosol, but in the presence of 1α,25(OH)2D3, VDR undergoes nuclear translocation. In order to stably associate with DNA at a VD response element (VDRE), VDR heterodimerizes with a retinoid X receptor (RXR; α, β, γ); multiple genome-wide ChIP-sequencing studies have confirmed co-localization of VDR with RXRs on the chromatin of associated sites.
The consensus VDRE is composed of 2 motifs, with the sequence 5′-AGGTCA, separated by 3 bp.[3,26,28] Even though the VDR-RXR heterodimer binds to VDREs, the transactivation function of VDR is silent in the absence of 1α,25(OH)2D3. The binding of 1α,25(OH)2D3 induces the ability of the VDR-RXR heterodimer to activate gene transcription.[29]
A number of transcriptional co-regulators, in addition to basal transcription factors, are involved in ligand-dependent activation of RNA polymerase II by VDR.[30,31] When transcriptionally silent unliganded VDR is bound to a VDRE, transcriptional co-repressors associate with VDR. Upon ligand binding, the co-repressors dissociate, and co-activators are recruited (Fig. 2).
Histone acetyltransferases (HATs) and histone deacetylases (HDACs) have been extensively characterized as co-regulators of the transcriptional activity of NRs.[30] Histone acetylation facilitates the transcriptional process by relaxing the nucleosome array and providing binding sites for proteins that associate with acetylated lysine. Accordingly, HATs act as co-activators for VDR and other NRs, while HDACs act as co-repressors. In addition, given the significance of methylation of lysine 4 and lysine 9 of histone H3, methyltransferases and demethylases that act on these 2 amino acids also influence the gene regulatory function of many NRs,[32,33] but they appear dispensable for gene regulation by VDR.
Among the NRs, sex hormone receptors interact with chromatin remodeling complexes, and chromatin reorganization influences the transcriptional regulation of these receptors.[34,35] However, VDR is unlikely to associate with chromatin remodeling complexes in the process of ligand-dependent gene regulation in most VD target cells. For example, one study employed Assay for Transposase-Accessible Chromatin sequencing (ATAC-seq) in the human keratinocyte cell line HaCaT and observed that chromatin organization is unaffected by the presence or absence of either 1α,25(OH)2D3 or VDR. Thus, the major transcriptional co-regulators for VDR are presumed to be enzymes related to histone acetylation.
VDR agonists have been generated in an effort to develop anti-osteoporotic therapies. One of the resulting weak agonists, Edirol®, has been widely applied with clinical success for the treatment of osteoporosis in Japan.[36,37] In other countries, VD is considered a dietary supplement rather than a drug, since VD has not been fully proven to be beneficial as an anti-osteoporotic therapy through extensive clinical studies.[7] The discrepancy in clinical views of VD between countries may be due in part to differences in the dietary intake of calcium.[21] As water tends to be softer (less calcemic) in Japan, it can be postulated that the anti-osteoporotic action of Edirol® is potentiated by calcium co-supplementation. Accordingly, VDR agonists like Edirol® may be more useful as anti-osteoporotic agents in areas where the water is soft and less dietary calcium is consumed.[4]
Given that VD insufficiency is closely related to the incidence of a number of diseases, the anti-inflammatory action of VD has been presumed to underlie its protective role in attenuating the onset of these diseases.[9,10] However, clinical trials have failed to prove the effectiveness of VD and its analogues in the treatment of prostate cancer, other cancers and related diseases. Since such VD analogues have been shown to significantly attenuate proliferation in cancer model cell lines, [18,20] failure of the clinical trials can be inferred to be due, at least in part, to the need to limit doses of VD analogues to avoid hypercalcemia.[21,22] Therefore, synthetic VD analogues that exert beneficial anti-inflammatory action in the absence of hypercalcemic activity are desired.
A related approach to the issue of hypercalcemia is the development of a VDR antagonist that would mitigate the hypercalcemic action of VD analogues without influencing the beneficial actions of VD for disease prevention. Recently, Rovito et al. [38] developed a VD analogue (ZK168281) to antagonize the action of VD. This compound partially trapped the VDR in the cytosol, and thereby antagonized VD action in gene regulation. The molecular basis for this activity appeared to involve differences in structural alterations to the ligand-binding domain (LBD) as induced upon binding of 1α,25(OH)2D3 and ZK168281 (Fig. 3). Interestingly, the hypercalcemic action induced by pharmacological doses of 1α,25(OH)2D3 in intact mice was blunted by this compound.[38] The authors proposed that this compound would have possible clinical applications for hypercalcemic patients, such as in cases of idiopathic infantile hypercalcemia, [25,26] but the potential for ZK168281 to attenuate inflammation remains to be studied.
The molecular mechanisms underlying the anti-inflammatory action of 1α,25(OH)2D3 remain unclear. However, the development of a partial VDR antagonist without its hypercalcemic action would be expected to realize the potential of the beneficial actions of 1α,25(OH)2D3, as administration of such a compound at higher doses would be possible. Full VDR antagonists appear detrimental to bone health, but a partial VDR antagonist could support bone health by not severely affecting calcium homeostasis.
Such an idea is supported by the clinal success of selective estrogen receptor (ER) modulators (SERMs) as anti-osteoporotic drugs.[39,40] The first generation SERM was raloxifene, and it has been administered to osteoporotic patients worldwide. Raloxifene was developed following epidemiological observations of patients with estrogen-dependent breast cancer, in which treatment with an ER-α antagonist (tamoxifen) unexpectedly did not negatively impact bone health; actually, some subjects experienced increases in bone mass. Importantly, tamoxifen had been considered at that time to be a full ER antagonist, [40] but these observations suggested that the definition of synthetic NR ligands, dependent on limited numbers of biological assays, is subject to error. Modern technologies then led to the clarification of the definition of synthetic NR ligands [41]; for example, a reporter gene assay that assessed the transactivation function of ER-α later showed that tamoxifen is an ER partial antagonist/antagonist.[42] Consistent with the findings of the molecular characterization of tamoxifen, raloxifene was also shown to be an ER partial antagonist/agonist. This drug supports calcium homeostasis in the musculoskeletal and cardiovascular systems while it exerts antagonistic activity in estrogen-dependent reproductive organs, including breast tissue and the uterus.[40]
Using similar therapeutic approaches, selective androgen receptor modulators (SARMs) have been generated, with the expectation that they would support the anabolic action of androgens in the musculoskeletal system but antagonize the action of androgens in proliferative cancer tissues.[43,44] Androgens serve as hormones that potently induce the production of muscle and bone mass, but over-production of androgen can serve as a major risk factor for androgen-dependent prostate cancer. The aberrant production of androgen can also lead to the development of male-associated characteristics in subjects who are genetically female. Thus, SARMs offer the attractive possibility of drugs that block the negative effects of androgens while supporting their positive effects. In particular, the clinical application of SARMs to older patients with sarcopenia is highly desired; such a strategy has not yet been successful in clinical trials.
Using the mechanisms of SERMs and SARMs as a model, we speculate that modern assays will be capable of identifying selective VDR ligands. Such ligands would act as agonists in the anti-inflammatory roles of VD while having a much weaker impact on the roles of VDR in calcium homeostasis. These assays thus would be capable of uncovering as-yet-unknown molecular functions of VD, and hence may constitute a foundation from which to screen for novel VDR-based therapies.
The tissue-specific actions of SERMs and SARMs have been inferred by molecular dissections of sex steroid receptors bound to synthetic ligands.[45,46] AR and the ERs form homodimers, and the monomers each possess 2 domains with ligand-dependent transactivation functions, the N-terminal activation function (AF) 1 and C-terminal AF2 domains. The AF2 domain is bifunctional, as it also serves as the LBD; it consists of 12 α-helical loops with a narrow hydrophobic pocket that specifically binds to the ligand.[35]
Upon ligand binding, α-helix 12, which is located near the C-terminal end of the protein, is drastically shifted, leading to structural alterations throughout the entire receptor. Such ligand-induced structural alterations of NR proteins lead to the dissociation of transcriptional co-repressors and the recruitment of co-activators, thus achieving ligand-induced transactivation.[33-35] As expression levels of transcriptional co-regulators are tissue- and cell-specific, ligand binding-dependent association and dissociation of transcriptional co-regulators occur in a cell-type-specific manner.[29,33] Consequently, the combinations of co-regulators that interact with the AF1 and AF2 domains of NRs in different cells reflect cell type-specific activities of these domains.
Structural analyses have shown that the binding of synthetic ligands shifts α-helix 12 of NRs to an angle distinct from that achieved upon cognate hormone binding, leading us to propose ligand type-specific conformational alterations over the entire receptor protein.[45,46] X-ray crystallographic analyses of the ER-α LBD bound to either raloxifene or estradiol provided the first such evidence,[47] and the findings correlated with the antagonistic action of raloxifene for ER-α AF2.[42] Similarly, the dominant function of AF1 over AF2 of ER-α in estrogenic action in bone tissue was demonstrated by using mouse mutants expressing only the ER-α AF1 or AF2 domain. The beneficial actions of raloxifene, presumably as well as those of the other SERMs, for bone were thus interpreted as resulting from the activation of ER-α AF1 (Table 1).
The molecular bases of the anabolic actions of androgens and SARMs largely remain elusive.[43,44] AR also has 2 functional domains, but the activity of AF2 is marginal when compared with that of AF1. Notably, the N-terminal A/B domain, which contains AF1, in AR is longer than that of ER-α.[35] This property of the ligand-induced transactivation function of AR may be an obstacle to the generation of tissue-specific SARMs.
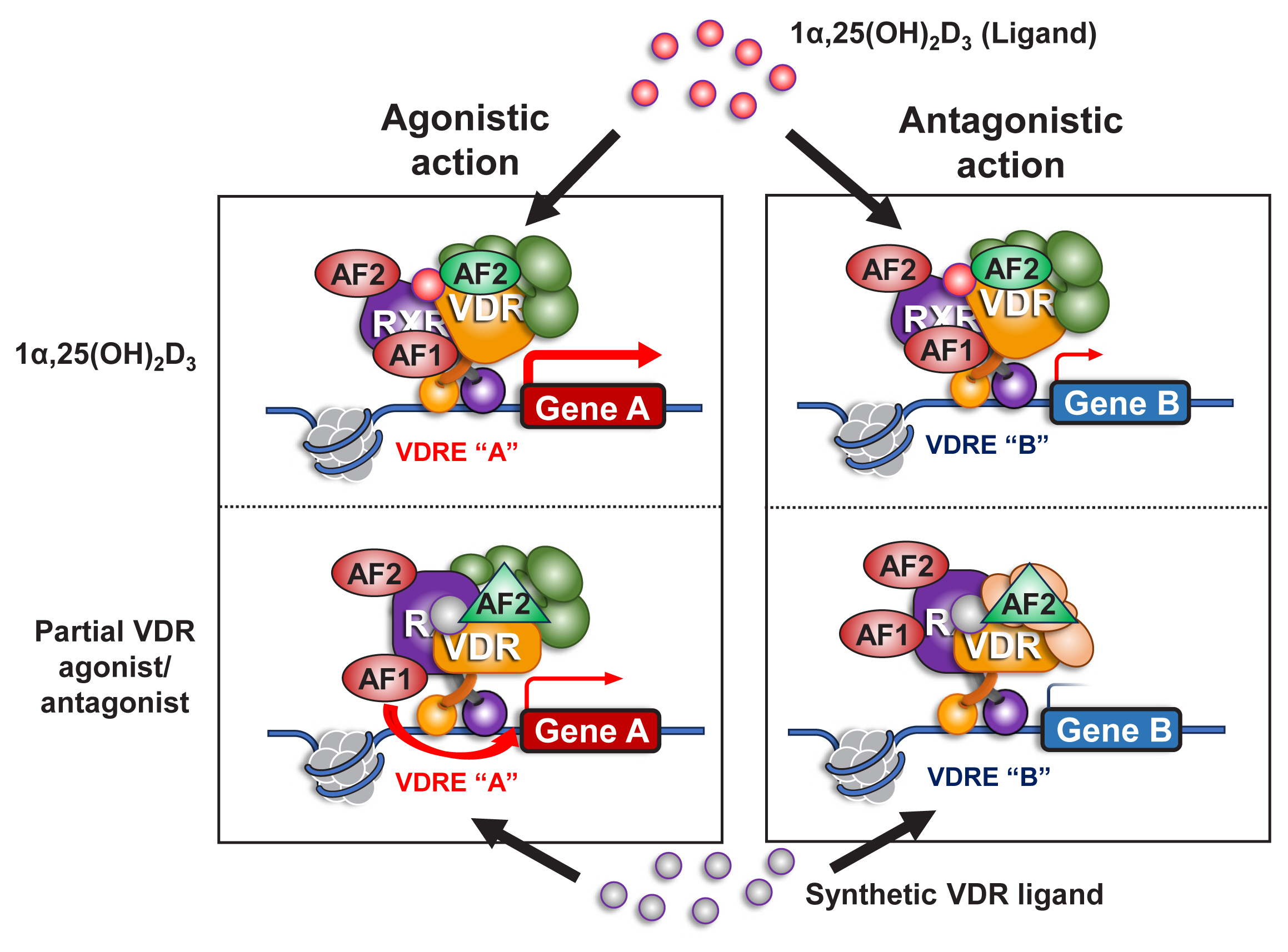
Unlike ER-α and AR, VDR contains a very short A/B domain, and this domain appears to lack a functional AF1 domain.[26,27] However, as VDR forms a heterodimer with one of the RXRs (RXRα, RXRβ, or RXRγ) to produce a ligand-dependent transactivation unit, the AF1 function of the partner RXR presumably facilitates the tissue-specific action of VD-liganded VDR.[48] It is feasible to generate a partial VDR antagonist or agonist with a minimal effect on hypercalcemia but that retains beneficial action in bone and other tissues (Fig. 4).
The desirable tissue-specific action of a VDR ligand would need to exert a different structural alteration of the VDR-containing heterodimer than that exerted by binding to endogenous 1α,25(OH)2D3. Such a possibility appears achievable in light of a recent report that showed that the structural alteration induced in the VDR LBD was different when ZK168281 or VD was bound.[38] In this regard, we recently aimed to develop a VDR antagonist, and we investigated the properties of the candidate molecule DLAM-2b. In human cell lines endogenously expressing VDR, the expected antagonistic action of DLAM-2b against VD with respect to the known VD target gene CYP24A1 was observed. By transcriptome analyses of the cultured human cells, DLAM-2b was also found to attenuate the 1α,25(OH)2D3-induced expression of a group of target genes; however, not all of the target genes known to be regulated by 1α,25(OH)2D3 were impacted. Moreover, DLAM-2b was found to alter the expression of genes that are not subject to regulation by 1α,25(OH)2D3, suggesting that VDR-bound DLAM-2b is able to interact with additional genes.
Since the altered structures of NRs bound to synthetic ligands have been shown to remodel chromatin organization, thereby leading to tissue-specific gene regulation,[35,49,50] we then used an ATAC-seq approach to compare chromatin remodeling in the presence of DLAM-2b or 1α,25(OH)2D3. However, chromatin reorganization was not observed in the cells treated with either of the 2 ligands; therefore, VDR is unlikely to be capable of chromatin remodeling. As the accessibility of the DNA at the activated gene loci was differentially regulated by the 2 ligands, histone modifications, such as acetylation and deacetylation, are more likely to facilitate gene regulation by the ligands. From these observations in human cells, we propose that DLAM-2b is a partial antagonist/antagonist for VDR, though an off-target effect not mediated by VDR on a certain set of regulated genes cannot be excluded. Moreover, further work is required to determine if DLAM-2b exerts tissue-specific actions related to 1α,25(OH)2D3-regulated events in intact animals.
VD exhibits a wide variety of biological actions, and dietary deficiency of VD underlies the incidence of multiple diseases. The molecular bases of VD action, as mediated by VDR, under normal conditions have been extensively studied, but the roles of VD and VDR in pathological settings remain largely unclear. As information regarding these systems emerges, and given the beneficial effects of VD in disease prevention, development of synthetic VD analogues with lowered hypercalcemic activity has become more promising. We expect that such compounds will be effective drugs for maintaining bone health while slowing the onset of diseases.
Acknowledgments
The authors thank all of the present and past members of laboratories who supported this study, and we thank Ms. Kayoko Wakamatsu for preparation of the manuscript.
Fig. 1
Schematic view of vitamin D (VD) signaling pathway mediated VD receptor (VDR). Active form of VD is biosynthesized from precursors taken from diet as well as endogenously generated cholesterol metabolites, and catabolized by P450 enzymes depicted in the figure. The key conversion into the active form of VD is conducted by cytochrome P450 (CYP)27B1, and the major catabolizing enzyme is CYP24A1. Active form of VD exerts biological action through gene regulation operated by VDR. Genetic mutations deficient of CYP27B1 and VDR function are causal hereditary rickets, similar to rachitic abnormality seen in rickets by nutritious deficiency of VD. Organs related with VD action are illustrated. UVB, ultraviolet B; 25(OH)D3, 25-hydroxy-vitamin D3; FGF23, fibroblast growth factor 23; PTH, parathyroid hormone; VDDR, vitamin D-dependent rickets; RXR, retinoid X receptor; VDR, vitamin D receptor; VDRE, vitamin D response element.
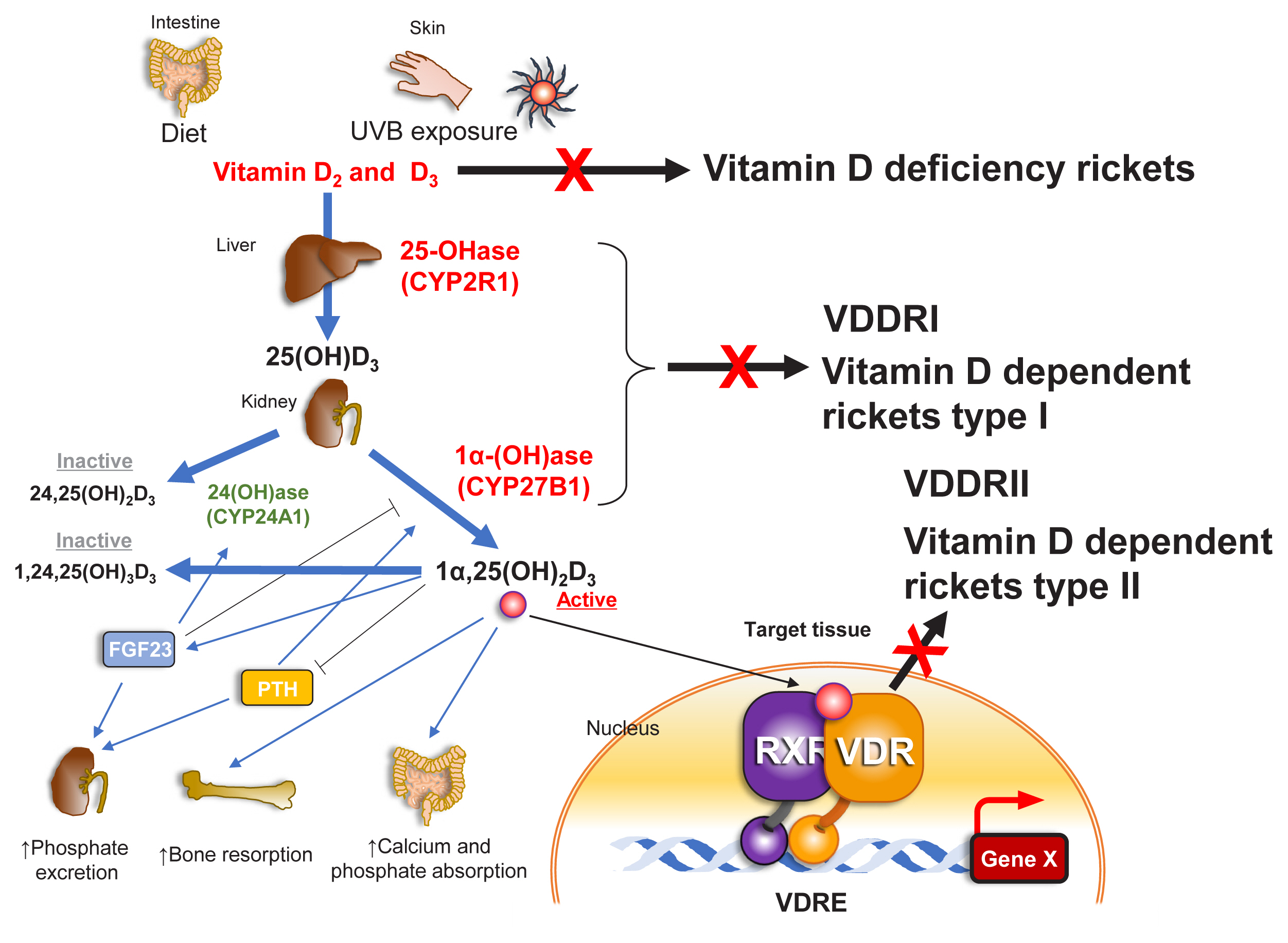
Fig. 2
Co-regulators for vitamin D receptor (VDR) in gene regulation. Chromatin environment is fundamental for gene regulation, and is organized during cell proliferation and differentiation. Chromatin remodeling complexes may directly and indirectly co-regulate VDR function on chromatin. Since VDR function in gene regulation is ligand binding-dependent, ligand-dependent association and dissociation of co-regulators are induced by structure alteration of VDR protein, presumably coupling with that of heterodimerized retinoid X receptor (RXR) protein. As VDR transactivates as well as transrepresses gene expression, both of co-activators and co-repressors are considered to acts as VDR co-regulators. For nuclear steroid receptors, various histone modifiers including histone methyltransferases (HMTs) and demethylases (HDMs) have been identified as co-regulators. However, unlike enzymes regulating histone acetylation (histone acetyltransferases [HATs] and histone deacetylases [HDACs]), either HMT or HDM looks unlikely to co-regulate VDR from the past reports. ATP, adenosine triphosphate; VDRE, vitamin D response element.
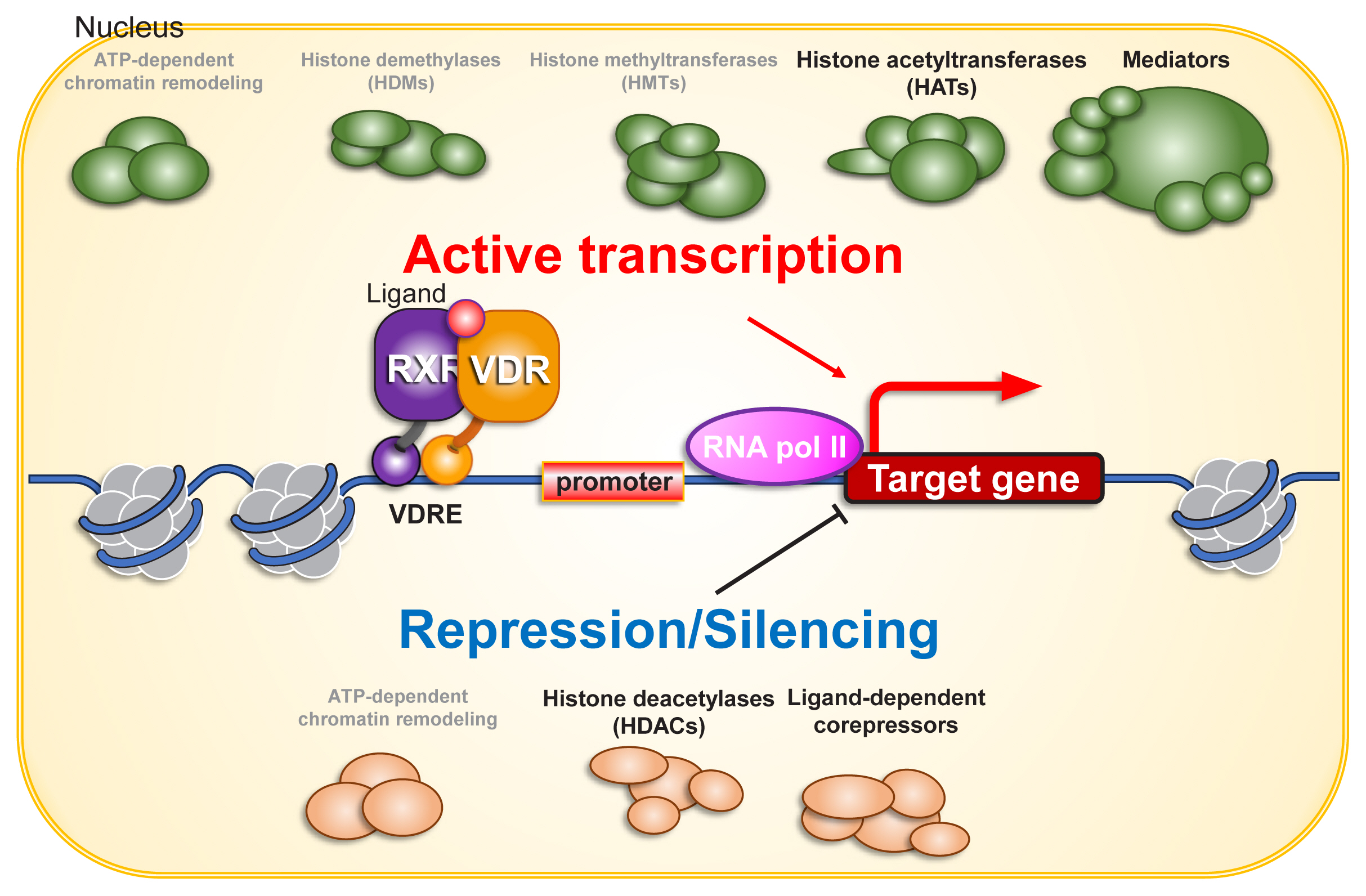
Fig. 3
Ligand-induced structural alteration of vitamin D receptor (VDR) protein underlies ligand-type-specific function of VDR in gene regulation. Ligand binding induces structural alteration of VDR protein, presumably affecting retinoid X receptor (RXR) protein structure. As the structural alteration is ligand-type specific, and ligand-type specific association and dissociation of VDR co-regulators result in ligand-type specific targeting on chromatin for gene regulation. 1α,25(OH)2D3, 1α,25-dihydroxy-vitamin D3; VDRE, vitamin D response element.
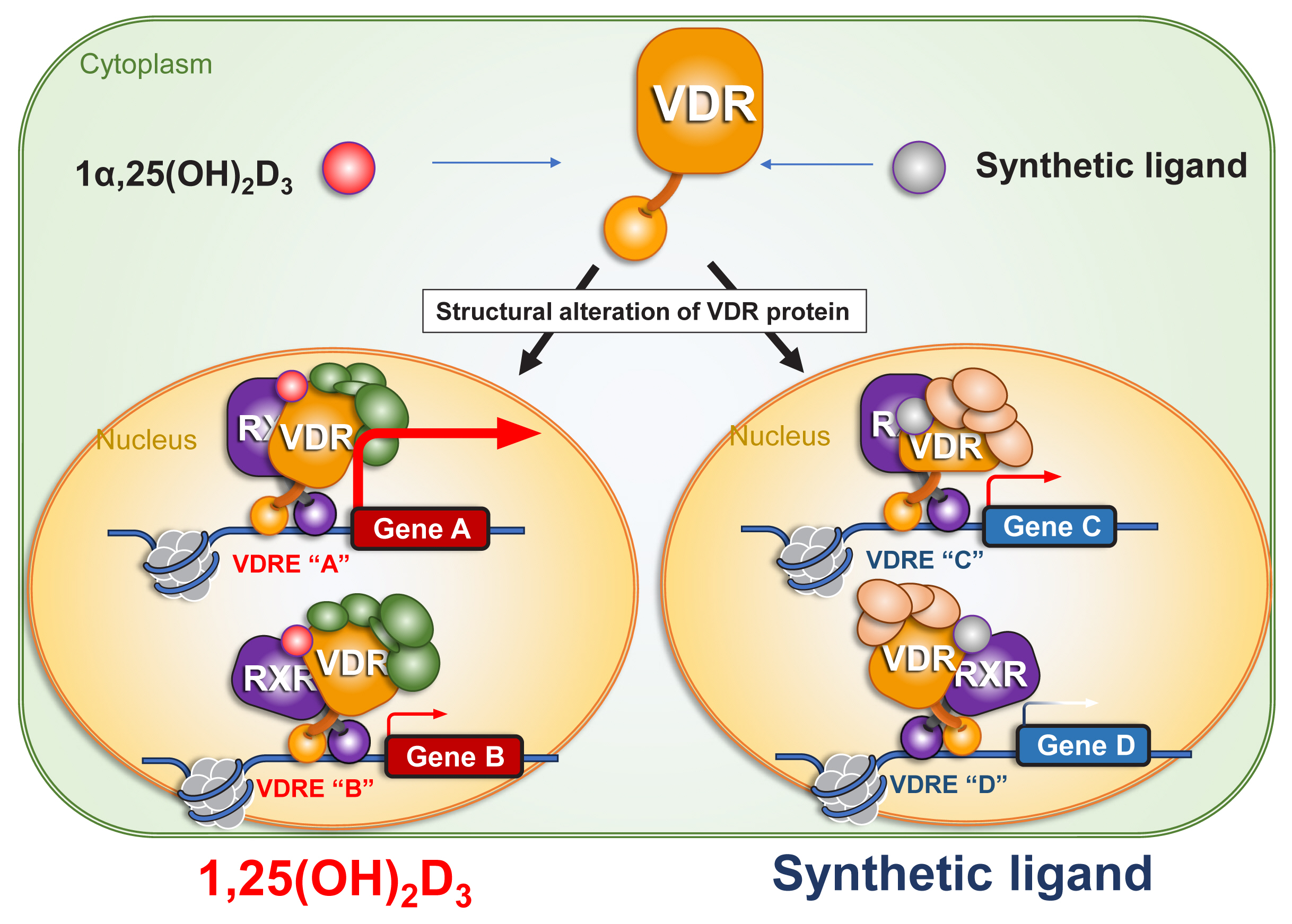
Fig. 4
A role of retinoid X receptor (RXR) in an agonistic and antagonistic action of a synthetic vitamin D receptor (VDR) ligand. VDR heterodimerizes with RXR as a functional unit in gene regulation on chromatin in response to a VDR ligand. VDR appears to encompass only 1 functional domain (activation function [AF] 2) at its C-terminal end for transcriptional control, but RXR has 2 functional domains (AF1 and AF2). A VDR antagonist antagonizes the VDR AF2 function, but may not be inhibitory for the AF1 function of RXR, thereby acting as a partial VDR antagonist/agonist. 1α,25(OH)2D3, 1α,25-dihydroxy-vitamin D3; VDRE, vitamin D response element.
REFERENCES
1. Morris HA, Anderson PH. Autocrine and paracrine actions of vitamin d. Clin Biochem Rev 2010;31:129-38.


2. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr 2008;87:1080s-6s. https://doi.org/10.1093/ajcn/87.4.1080S.


3. Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev 2008;29:726-76. https://doi.org/10.1210/er.2008-0004.



4. Yoshizawa T, Handa Y, Uematsu Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 1997;16:391-6. https://doi.org/10.1038/ng0897-391.


5. Kitanaka S, Takeyama K, Murayama A, et al. Inactivating mutations in the 25-hydroxyvitamin D3 1alpha-hydroxylase gene in patients with pseudovitamin D-deficiency rickets. N Engl J Med 1998;338:653-61. https://doi.org/10.1056/nejm199803053381004.


6. Panda DK, Miao D, Tremblay ML, et al. Targeted ablation of the 25-hydroxyvitamin D 1alpha-hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci USA 2001;98:7498-503. https://doi.org/10.1073/pnas.131029498.



7. Zhang Y, Fang F, Tang J, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ 2019;366:l4673.https://doi.org/10.1136/bmj.l4673.



8. Bouillon R, Manousaki D, Rosen C, et al. The health effects of vitamin D supplementation: evidence from human studies. Nat Rev Endocrinol 2022;18:96-110. https://doi.org/10.1038/s41574-021-00593-z.



9. Nielsen OH, Hansen TI, Gubatan JM, et al. Managing vitamin D deficiency in inflammatory bowel disease. Frontline Gastroenterol 2019;10:394-400. https://doi.org/10.1136/flgastro-2018-101055.



10. Samuel S, Sitrin MD. Vitamin D's role in cell proliferation and differentiation. Nutr Rev 2008;66:S116-24. https://doi.org/10.1111/j.1753-4887.2008.00094.x.


11. Miyamoto H, Kawakami D, Hanafusa N, et al. Determination of a serum 25-hydroxyvitamin D reference ranges in Japanese adults using fully automated liquid chromatography-tandem mass spectrometry. J Nutr 2023;153:1253-64. https://doi.org/10.1016/j.tjnut.2023.01.036.


12. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 2019;51:27-41. https://doi.org/10.1016/j.immuni.2019.06.025.



13. Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019;25:1822-32. https://doi.org/10.1038/s41591-019-0675-0.



14. Forno E, Bacharier LB, Phipatanakul W, et al. Effect of vitamin D3 supplementation on severe asthma exacerbations in children with asthma and low vitamin D levels: the VDKA randomized clinical trial. JAMA 2020;324:752-60. https://doi.org/10.1001/jama.2020.12384.



15. Harrison SR, Li D, Jeffery LE, et al. Vitamin D, autoimmune disease and rheumatoid arthritis. Calcif Tissue Int 2020;106:58-75. https://doi.org/10.1007/s00223-019-00577-2.



16. Sherman MH, Yu RT, Engle DD, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014;159:80-93. https://doi.org/10.1016/j.cell.2014.08.007.



17. Wei Z, Yoshihara E, He N, et al. Vitamin D switches BAF complexes to protect β cells. Cell 2018;173:1135-49e15. https://doi.org/10.1016/j.cell.2018.04.013.



18. Leyssens C, Verlinden L, Verstuyf A. The future of vitamin D analogs. Front Physiol 2014;5:122.https://doi.org/10.3389/fphys.2014.00122.



19. Harrison JS, Bershadskiy A. Clinical experience using vitamin d and analogs in the treatment of myelodysplasia and acute myeloid leukemia: a review of the literature. Leuk Res Treatment 2012;2012:125814.https://doi.org/10.1155/2012/125814.



20. Manson JE, Cook NR, Lee IM, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 2019;380:33-44. https://doi.org/10.1056/NEJMoa1809944.



21. Yin K, Agrawal DK. Vitamin D and inflammatory diseases. J Inflamm Res 2014;7:69-87. https://doi.org/10.2147/jir.S63898.



22. Chen Y, Zhang J, Ge X, et al. Vitamin D receptor inhibits nuclear factor κB activation by interacting with IκB kinase β protein. J Biol Chem 2013;288:19450-8. https://doi.org/10.1074/jbc.M113.467670.



23. Christakos S, Dhawan P, Verstuyf A, et al. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev 2016;96:365-408. https://doi.org/10.1152/physrev.00014.2015.



24. Schlingmann KP, Kaufmann M, Weber S, et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med 2011;365:410-21. https://doi.org/10.1056/NEJMoa1103864.


25. Tebben PJ, Milliner DS, Horst RL, et al. Hypercalcemia, hypercalciuria, and elevated calcitriol concentrations with autosomal dominant transmission due to CYP24A1 mutations: effects of ketoconazole therapy. J Clin Endocrinol Metab 2012;97:E423-7. https://doi.org/10.1210/jc.2011-1935.



26. Haussler MR, Whitfield GK, Haussler CA, et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res 1998;13:325-49. https://doi.org/10.1359/jbmr.1998.13.3.325.


27. Sawatsubashi S, Nishimura K, Mori J, et al. The function of the vitamin D receptor and a possible role of enhancer RNA in epigenomic regulation of target genes: implications for bone metabolism. J Bone Metab 2019;26:3-12. https://doi.org/10.11005/jbm.2019.26.1.3.



28. Takeyama K, Kitanaka S, Sato T, et al. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science 1997;277:1827-30. https://doi.org/10.1126/science.277.5333.1827.


29. Meyer MB, Goetsch PD, Pike JW. A downstream intergenic cluster of regulatory enhancers contributes to the induction of CYP24A1 expression by 1alpha,25-dihydroxyvitamin D3. J Biol Chem 2010;285:15599-610. https://doi.org/10.1074/jbc.M110.119958.



30. Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev 2000;14:121-41.


31. Kato S, Yokoyama A, Fujiki R. Nuclear receptor coregulators merge transcriptional coregulation with epigenetic regulation. Trends Biochem Sci 2011;36:272-81. https://doi.org/10.1016/j.tibs.2011.01.001.


32. Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet 2012;13:343-57. https://doi.org/10.1038/nrg3173.



33. Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev 2006;20:1405-28. https://doi.org/10.1101/gad.1424806.


34. Chen T, Dent SY. Chromatin modifiers and remodellers: regulators of cellular differentiation. Nat Rev Genet 2014;15:93-106. https://doi.org/10.1038/nrg3607.



35. Sawada T, Kanemoto Y, Kurokawa T, et al. The epigenetic function of androgen receptor in prostate cancer progression. Front Cell Dev Biol 2023;11:1083486.https://doi.org/10.3389/fcell.2023.1083486.



36. Matsumoto T, Yamamoto K, Takeuchi T, et al. Eldecalcitol is superior to alfacalcidol in maintaining bone mineral density in glucocorticoid-induced osteoporosis patients (e-GLORIA). J Bone Miner Metab 2020;38:522-32. https://doi.org/10.1007/s00774-020-01091-4.


37. Matsumoto T, Ito M, Hayashi Y, et al. A new active vitamin D3 analog, eldecalcitol, prevents the risk of osteoporotic fractures-a randomized, active comparator, double-blind study. Bone 2011;49:605-12. https://doi.org/10.1016/j.bone.2011.07.011.


38. Rovito D, Belorusova AY, Chalhoub S, et al. Cytosolic sequestration of the vitamin D receptor as a therapeutic option for vitamin D-induced hypercalcemia. Nat Commun 2020;11:6249.https://doi.org/10.1038/s41467-020-20069-4.



39. Riggs BL, Hartmann LC. Selective estrogen-receptor modulators - mechanisms of action and application to clinical practice. N Engl J Med 2003;348:618-29. https://doi.org/10.1056/NEJMra022219.


40. Jordan VC. Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell 2004;5:207-13. https://doi.org/10.1016/s1535-6108(04)00059-5.

41. McDonnell DP, Wardell SE. The molecular mechanisms underlying the pharmacological actions of ER modulators: implications for new drug discovery in breast cancer. Curr Opin Pharmacol 2010;10:620-8. https://doi.org/10.1016/j.coph.2010.09.007.

42. Berry M, Metzger D, Chambon P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J 1990;9:2811-8. https://doi.org/10.1002/j.1460-2075.1990.tb07469.x.



43. Negro-Vilar A. Selective androgen receptor modulators (SARMs): a novel approach to androgen therapy for the new millennium. J Clin Endocrinol Metab 1999;84:3459-62. https://doi.org/10.1210/jcem.84.10.6122.


44. Solomon ZJ, Mirabal JR, Mazur DJ, et al. Selective androgen receptor modulators: current knowledge and clinical applications. Sex Med Rev 2019;7:84-94. https://doi.org/10.1016/j.sxmr.2018.09.006.



45. Nettles KW, Sun J, Radek JT, et al. Allosteric control of ligand selectivity between estrogen receptors alpha and beta: Implications for other nuclear receptors. Mol Cell 2004;13:317-27. https://doi.org/10.1016/s1097-2765(04)00054-1.


46. Nadal M, Prekovic S, Gallastegui N, et al. Structure of the homodimeric androgen receptor ligand-binding domain. Nat Commun 2017;8:14388.https://doi.org/10.1038/ncomms14388.



47. Brzozowski AM, Pike AC, Dauter Z, et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 1997;389:753-8. https://doi.org/10.1038/39645.


48. Nagpal S, Saunders M, Kastner P, et al. Promoter context- and response element-dependent specificity of the transcriptional activation and modulating functions of retinoic acid receptors. Cell 1992;70:1007-19. https://doi.org/10.1016/0092-8674(92)90250-g.

49. Lupien M, Eeckhoute J, Meyer CA, et al. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell 2008;132:958-70. https://doi.org/10.1016/j.cell.2008.01.018.



50. Sawada T, Kanemoto Y, Amano R, et al. Antagonistic action of a synthetic androgen ligand mediated by chromatin remodeling in a human prostate cancer cell line. Biochem Biophys Res Commun 2022;612:110-8. https://doi.org/10.1016/j.bbrc.2022.04.109.


- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 7,206 View
- 60 Download
- ORCID iDs
-
Yoshiaki Kanemoto

https://orcid.org/0000-0002-0270-180XTakahiro Sawada

https://orcid.org/0009-0002-2194-2681Tomohiro Kurokawa

https://orcid.org/0000-0002-7932-8724Kazuo Nagasawa

https://orcid.org/0000-0002-0437-948XShigeaki Kato

https://orcid.org/0000-0002-5564-1695 - Related articles




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print