|
 |
| jbm > Volume 26(1); 2019 > Article |
|
Abstract
Background
This study examined the change in the trabecular bone score (TBS), areal bone mineral density (aBMD), and osteoporosis in postmenopausal women who underwent thyrotropin (TSH)-suppressive therapy for treating papillary thyroid cancer after a total thyroidectomy procedure.
Methods
We evaluated 36 postmenopausal women who received a total thyroidectomy for papillary thyroid cancer and were undergoing TSH suppressive therapy with levothyroxine. Postmenopausal women (n=94) matched for age and body mass index were recruited as healthy controls. The aBMD and TBS of the lumbar spine were compared between dual energy X-ray absorptiometry (DXA) at baseline and at follow-up after an average of 4.92 years.
Results
There was no significant difference in the rate of diagnoses of osteoporosis, osteopenia, or normal bone status between the 2 groups during the baseline DXA evaluation. However, the TBS was significantly lower whereas aBMD did not show significant difference at the time of baseline DXA measurement (1st DXA, 1.343±0.098 vs. 1.372±0.06317, P<0.001; 2nd DXA, 1.342±0.095 vs. 1.370±0.062, P<0.001). The TBS and aBMD did not differ significantly between the initial and follow-up DXA images in both groups of TSH suppressive patients and controls.
Among many endocrinological causes of secondary osteoporosis (e.g., Cushing's syndrome, hyperparathyroidism, hypogonadism), conditions of excess or deficient thyrotropin (thyroid-stimulating hormone [TSH]) and thyroid hormones are potentially damaging to bone status.[1,2] Although endogenous hyperthyroidism is associated with an increased risk of osteoporosis due to increased osteoclastic resorption and decreased bone formation,[3,4] the effect of suppressive thyroxine therapy on osteoporosis remains unclear. Recent concerns have emerged that lifelong TSH-suppressive therapy may have the potentially harmful effects on the heart and skeleton induced by subclinical hyperthyroidism [5] differentiated thyroid cancer (DTC) is the most common endocrine malignancy and is generally an indolent tumor with a 10-year overall survival rate of 80% to 95%.[6]
Osteoporosis is the recent concern regarding lifelong TSH-suppressive therapy.[5,7,8,9,10,11] Osteoporosis is a major healthcare challenge worldwide; the number of newly diagnosed cases and associated costs of osteoporotic fracture are increasing with age. Both menopause and hyperthyroidism are the risk factor of osteoporosis,[12] and women with hyperthyroidism experience their first fractures earlier during life than do those without.[13] Recent studies reported that, suppressed TSH level is significantly correlated with the risk of osteoporosis after total thyroidectomy.[14,15] Osteoporosis is characterized by a low bone mass and microarchitectural deterioration. The World Health Organization's standard criterion for diagnosing osteoporosis uses densitometry tools, such as dual energy X-ray absorptiometry (DXA) for measuring areal bone mineral density (aBMD; mg/cm2) and calculating a T-score. The trabecular bone score (TBS) is a new textural index obtained from DXA, that evaluates pixel gray-level variations of the lumbar spines has recently been proposed as an representative parameter for the trabecular bone microarchitecture independent of BMD.[16] According to recent study, Recuded TBS has association with increased osteoporotic vertebral fractures risk in patients with osteopenia, as well as in those with normal BMD.[17] In healthy euthyroid post-menopausal women, the TBS is negatively correlated with free thyroxine (fT4) levels within range for high-to-normal.[18] In postmenopausal women with DTC, a longer duration of TSH suppression was independently associated with a decreased lumbar spine TBS.[19] From a clinical perspective, the additional information about bone quality provided by the TBS could aid the management of patients with osteopenia, as well as those with normal BMD who have risk factors for osteoporosis.
We designed this retrospective study to determine whether suppressive L-thyroxine (LT4) therapy after a total thyroidectomy influences the TBS, and compare the incidence of osteoporosis with a healthy control group.
This retrospective study included postmenopausal women with DTC who underwent a total thyroidectomy and began follow-up testing between October 2013 and January 2017 at Hospital. The strict selection criteria for the TSH-suppression group were as follows: (1) no distant metastasis or invasion of locoregional structures; (2) postmenopausal status with a baseline DXA evaluation; (3) treatment with LT4 at suppressive doses to maintain a serum TSH level <0.3 mU/I; (4) fT4 level within the normal range; (5) free of thyroid cancer on clinical and laboratory examinations during follow-up (negative neck ultrasound and undetectable serum thyroglobulin); and (6) regular follow-up visits. Because menopause is a risk factor for osteoporosis, all patients underwent repeat DXA for evaluation of bone mineral density. We enrolled postmenopausal women (n=36) with papillary thyroid cancer who underwent total thyroidectomy and TSH suppression.
To identify the healthy controls, we enrolled postmenopausal women (n=94) who visited our hospital for a health check-up and matched in age and body mass index (BMI) to the treatment group. They had annual checkup for lumbar spine DXA and thyroid function tests (serum TSH, fT4, and T3). The participants in the healthy control group had serum TSH levels within the normal reference range and did not have hypo- or hyperthyroidism or a history of malignant disease. Exclusion criteria for all participants included renal or hepatic impairment and exposure to any medication that affects bone metabolism such as glucocorticoids or anti-osteoporotic drugs.
TSH and fT4 levels were measured using the conventional BRAHMS manual immunoradiometric assay (Thermo Fisher Scientific BRAHMS GmbH, Hennigsdorf, Germany). Laboratory analyses of calcium, phosphate, and alkaline phosphatase levels were performed on the same day as DXA.
This retrospective study was approved by our Institutional Review Board (IRB), which waived the requirement for written consent (IRB No. H-1711-008-061).
The aBMD of the lumbar vertebrae (L1-4) was measured on DXA scans (Lunar Prodigy; GE Medical Systems, Milwaukee, WI, USA) and analyzed using Encore software (version 13.0; GE Healthcare, Madison, WI, USA). Calibration and quality assurance tests were performed daily. The coefficient of variation for precise measurement of the aBMD of the lumbar spine was 0.34%. T-scores were defined according to the number of standard deviations (SDs) from the mean BMD of a reference group from the general population aged 25 to 35 years and matched for sex, as follows: l normal, lowest T-score≥−1.0; osteopenia, −2.5<lowest T-score<−1.0; and osteoporosis, ≤−2.5. The TBS of the lumbar spine was extrapolated from the lumbar spine DXA file using iNsight software (version 3.0.2.0; Medimaps, Merignac, France). A TBS was classified by a cutoff of >1.350 for a normal TBS value according to an international working group of TBS users for postmenopausal women.[20] The ratios of TBS and aBMD deterioration were calculated according to the baseline and final follow-up values.
All normally distributed variables were expressed as means±SD, while variables with non-normal distributions are expressed as medians and interquartile ranges (IQRs; 25−75%). To compare categorical data between the treatment and control groups, the χ2 test was used. A paired t-test was used to compare the follow-up DXA scans. The Mann-Whitney U test was used to compare the median values of non-normally distributed variables between the treatment and control groups. P-values <0.05 were considered statistically significant. The statistical analyses were performed using both MedCalc® for Windows software (version 16.4.3; MedCalc, Mariakerke, Belgium) and GraphPad Prism® (version 6; GraphPad Software, La Jolla, CA, USA).
There was no difference in the median follow-up period from the baseline DXA evaluation between the TSH suppression group (4.0±1.7 years) and the control group (4.4±1.7 years; P=0.923). The median age (60.5±5.5 years vs. 60.8±5.5 years, P=0.127) and BMI (23.31±2.77 vs. 23.20±2.93, P=0.356) also did not differ between the TSH suppression and control groups. The TSH-suppressive therapy was maintained for 4.66±1.52 years after total thyroidectomy at the baseline DXA study. The daily dose of LT4 for the patient group was 200 to 750 µg/day or 2.500±0.774 µg/kg. Of the patients in the TSH suppression group, 32 (88.89%) received calcium/vitamin D supplementation. Of the participants in the control group, 34 (36.17%) received calcium/vitamin D supplementation. The TSH levels of all patients in the TSH suppression group were nearly non-detectable (median, 0.000; IQR, 0.000-0.254), whereas those in the control group were within the normal range (median, 1.975; IQR, 1.141-1.644; P<0.001). The fT4 levels of all participants were within the normal range; however, the mean level differed significantly between the TSH suppression group (1.706±0.319) and control group (1.240±0.165; P<0.001). The baseline clinical characteristics of the participants in both groups are shown in Tables 1 and 2.
There was no significant difference in the rate of diagnosis of osteoporosis, osteopenia, or normal bone status between the 2 groups during the baseline DXA evaluation (Fig. 1). The average aBMD of the patients who received TSH-suppressive therapy was not significantly different from that of the control group (1st DXA, 0.997±0.149 vs. 1.081±0.148, P=0.415; 2nd DXA, 0.986±0.145 vs. 1.074±0.147, P=0.670; Table 2) and did not change significantly during follow-up (Fig. 2A). The mean TBS at the 1st DXA and follow-up DXA evaluations was significantly lower in patients with TSH suppression than in the control group (1st DXA, 1.344±0.098 vs. 1.372±0.063, P<0.001; 2nd DXA, 1.342±0.095 vs. 1.370±0.062, P<0.001; Table 2) whereas TBS was not significantly changed comparing at baseline and follow-up regardless of TSH suppressive treatment (Fig. 2B). According to the TBS threshold of 1.350, the status of the microarchitecture was not significantly different between the 2 groups on the follow-up scans.
After a total thyroidectomy, exogenous LT4 therapy for suppression of TSH and radio-iodine ablation therapy have been considered the standard treatments for DTC.[21,22,23] These treatments reduce the risk of tumor recurrence and improve patient survival.[6,24] A meta-analysis by McGriff et al.[25] reported that TSH-suppressive therapy decreased the risk of major adverse clinical events, including disease progression, recurrence, and death. Despite studies showing that TSH suppressive therapy improves patient survival, there is increasing concern about the adverse effects of lifelong therapy, because DTC patients have a long life expectancy after surgery.
The critical clinical perspective on bone status has emerged largely because of the extended survival and resulted long-term subclinical hyperthyroid state. Since Ross et al.[11] reported that long-term exposure to exogenous thyroid hormone was associated with bone loss, studies had reported that TSH-suppressive therapy may cause a significant deterioration of BMD in older patients.[14,26,27,28] However, the effect of exogenous TSH-suppressive therapy remains under debate. Heemstra et al.[28] suggested that postmenopausal women undergoing long-term TSH-suppressive therapy suffer from a lower BMD and higher risk of osteoporosis compared with controls. In other studies, the change in the BMD of postmenopausal patients with DTC on suppressive therapy was unclear,[29] or there were no significant effect of long-term TSH-suppressive therapy on BMD or bone turnover markers.[30,31]
The result of this study revealed the average aBMD of the patients who received TSH-suppressive therapy was not significantly decreased than that of the control group during follow-up. It coincided with report of Kim et al.[32], the study of 100 postmenopausal women and 24 premenopausal women on TSH suppression LT4 therapy, there was no significant change of BMD and bone turnover marker. The TBS decreased during the follow-up DXA evaluation over a mean of 4.02 years without statistical significance. Although there was no significant change of TBS, there was significant difference between patients and control at the time of baseline DXA measurement. One study reported that the TBS of the lumbar spine was independently and negatively correlated with the duration of TSH-suppressive therapy in DTC patients.[19] Our data were adjusted for age, BMI, and follow-up period, but we could not find the correlation between the TBS and TSH suppression duration. We expect that patients on TSH suppression will have a worse TBS at earlier period of TSH suppression, and no more aggravation after that, compared with healthy menopausal women, as described by Moon et al.[19].
DXA considered as the reference standard method for determining bone density and commonly sued way of monitoring treatment efficacy in clinical settings;[33] however, DXA has limited ability to evaluate BMD or bone quality. The TBS provides indirect indices of the trabecular microarchitecture [34] and changes prior to the T-score of DXA. TBS additive value BMDs in terms of the properties of a degraded microarchitecture of the lumbar spine than are traditional density measurements.[16] A low TBS significantly increases the risk of fragility fractures independent of BMD. [35] In particular, the TBS has been shown to be better in identifying in subjects with osteoporotic whose BMDs were normal or osteopenia on DXA with degraded microarchitecture and vertebral fractures.[17]
TSH is hypothesized to promote osteoblast differentiation by enhancing alkaline phosphatase levels and preventing bone loss, leading to skeletal remodeling and improved microarchitecture according to preclinical studies.[36] Additionally, a high level of fT4 causes skeletal toxicity, while triiodothyronine stimulates osteoclasts that increase bone turnover.[37] However, the significant difference only appeared at the early DXA measurement and there was no significant change during long term follow up period of median 4.66 years. We can infer that TSH suppression using an excessive dose of LT4 may have a negative effect on skeletal remodeling immediately after total thyroidectomy. Moreover, menopause is a major risk factor for osteoporosis. Therefore, post-menopausal women with a high-to-normal fT4 level undergoing TSH suppression with LT4 therapy experience more severe distress from a decrease in BMD in early stage of TSH suppressive therapy.
Based on our analysis, we suggest that concerns about osteoporosis in DTC patients can identify low TBS, reduce fracture risk and improve quality of life. Furthermore, we strongly advise an in-depth assessment of bone density with TBS in postmenopausal patients with DTC undergoing TSH-suppressive LT4 therapy, especially in the early stage of TSH suppressive therapy. This management process is essential because long-term TSH-suppressive LT4 therapy in postmenopausal period will unavoidably affect to further decrease in BMD.[18,19,28,38]
Our study had several limitations, including a relatively small patient population. The retrospective study design did not allow us to confirm the laboratory findings of bone turnover markers that could explain the participants' bone metabolism or to review the radiologic images for evaluation of fragility fractures during follow-up or to identify other medical history affecting bone mass and quality. Because of the critical period of bone loss starting at menopause, the unknown menopausal status during total thyroidectomy is another potential limitation.
In conclusion, the results of our study have clinical implications for and evaluating TBS in menopausal patients on long-term TSH-suppressive therapy. More prospective studies of TBS and its relationship with the risk of fracture, and multivariate analysis for the identification of risk factors for progression to osteoporosis, are needed to confirm our findings.
ACKNOWLEDGMENTS
Keunyoung Kim was supported by the Bio & Medical Technology Development Program of the NRF funded by the Korean government, MSIP (No. 2018M3A9E8066252).
DECLARATIONS
AUTHOR CONTRIBUTION:
Conceptualization: Kim IJ, Jeon YK, Kim K.
Data curation: Goh TS, Shin S, Lee JG, Park K.
Formal analysis: Kim K, Kim EH.
Investigation: Goh TS, Shin S, Lee JG, Park K.
Methodology: Kim SJ, Kim BH, Kim SS, Lee BJ.
Software: Park K.
Validation: Kim IJ, Jeon YK, Kim SJ, Kim BH, Kim SS, Lee BJ.
Writing - original draft: Kim K, Kim EH.
Writing - review & editing: Jeon YK, Kim K.
References
1. Abe E, Marians RC, Yu W, et al. TSH is a negative regulator of skeletal remodeling. Cell 2003;115:151-162.


2. Sendak RA, Sampath TK, McPherson JM. Newly reported roles of thyroid-stimulating hormone and follicle-stimulating hormone in bone remodelling. Int Orthop 2007;31:753-757.




3. Eriksen EF, Mosekilde L, Melsen F. Trabecular bone remodeling and bone balance in hyperthyroidism. Bone 1985;6:421-428.


4. Mosekilde L, Melsen F, Bagger JP, et al. Bone changes in hyperthyroidism: interrelationships between bone morphometry, thyroid function and calcium-phosphorus metabolism. Acta Endocrinol (Copenh) 1977;85:515-525.


5. Biondi B, Cooper DS. Benefits of thyrotropin suppression versus the risks of adverse effects in differentiated thyroid cancer. Thyroid 2010;20:135-146.


6. Cady B, Cohn K, Rossi RL, et al. The effect of thyroid hormone administration upon survival in patients with differentiated thyroid carcinoma. Surgery 1983;94:978-983.

7. Klein Hesselink EN, Klein Hesselink MS, de Bock GH, et al. Long-term cardiovascular mortality in patients with differentiated thyroid carcinoma: an observational study. J Clin Oncol 2013;31:4046-4053.


8. Flynn RW, Bonellie SR, Jung RT, et al. Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J Clin Endocrinol Metab 2010;95:186-193.


9. de Melo TG, da Assumpção LV, Santos Ade O, et al. Low BMI and low TSH value as risk factors related to lower bone mineral density in postmenospausal women under levothyroxine therapy for differentiated thyroid carcinoma. Thyroid Res 2015;8:7




10. Bauer DC, Ettinger B, Nevitt MC, et al. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med 2001;134:561-568.


11. Ross DS, Neer RM, Ridgway EC, et al. Subclinical hyperthyroidism and reduced bone density as a possible result of prolonged suppression of the pituitary-thyroid axis with L-thyroxine. Am J Med 1987;82:1167-1170.


13. Solomon BL, Wartofsky L, Burman KD. Prevalence of fractures in postmenopausal women with thyroid disease. Thyroid 1993;3:17-23.


14. Grimnes G, Emaus N, Joakimsen RM, et al. The relationship between serum TSH and bone mineral density in men and postmenopausal women: the Tromso study. Thyroid 2008;18:1147-1155.


15. Wang LY, Smith AW, Palmer FL, et al. Thyrotropin suppression increases the risk of osteoporosis without decreasing recurrence in ATA low- and intermediate-risk patients with differentiated thyroid carcinoma. Thyroid 2015;25:300-307.



16. Pothuaud L, Carceller P, Hans D. Correlations between greylevel variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone 2008;42:775-787.


17. Lee JE, Kim KM, Kim LK, et al. Comparisons of TBS and lumbar spine BMD in the associations with vertebral fractures according to the T-scores: a cross-sectional observation. Bone 2017;105:269-275.


18. Hwangbo Y, Kim JH, Kim SW, et al. High-normal free thyroxine levels are associated with low trabecular bone scores in euthyroid postmenopausal women. Osteoporos Int 2016;27:457-462.


19. Moon JH, Kim KM, Oh TJ, et al. The effect of TSH suppression on vertebral trabecular bone scores in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab 2017;102:78-85.


20. Silva BC, Bilezikian JP. Trabecular bone score: perspectives of an imaging technology coming of age. Arq Bras Endocrinol Metabol 2014;58:493-503.



21. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-1214.


22. Biondi B, Filetti S, Schlumberger M. Thyroid-hormone therapy and thyroid cancer: a reassessment. Nat Clin Pract Endocrinol Metab 2005;1:32-40.



23. Pacini F, Schlumberger M, Dralle H, et al. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol 2006;154:787-803.


24. Balme HW. Metastatic carcinoma of the thyroid successfully treated with thyroxine. Lancet 1954;266:812-813.


25. McGriff NJ, Csako G, Gourgiotis L, et al. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med 2002;34:554-564.

26. Sugitani I, Fujimoto Y. Effect of postoperative thyrotropin suppressive therapy on bone mineral density in patients with papillary thyroid carcinoma: a prospective controlled study. Surgery 2011;150:1250-1257.


27. Kung AW, Yeung SS. Prevention of bone loss induced by thyroxine suppressive therapy in postmenopausal women: the effect of calcium and calcitonin. J Clin Endocrinol Metab 1996;81:1232-1236.

28. Heemstra KA, Hamdy NA, Romijn JA, et al. The effects of thyrotropin-suppressive therapy on bone metabolism in patients with well-differentiated thyroid carcinoma. Thyroid 2006;16:583-591.


29. Quan ML, Pasieka JL, Rorstad O. Bone mineral density in well-differentiated thyroid cancer patients treated with suppressive thyroxine: a systematic overview of the literature. J Surg Oncol 2002;79:62-69.


30. Lee MY, Park JH, Bae KS, et al. Bone mineral density and bone turnover markers in patients on long-term suppressive levothyroxine therapy for differentiated thyroid cancer. Ann Surg Treat Res 2014;86:55-60.



31. Franklyn JA, Betteridge J, Daykin J, et al. Long-term thyroxine treatment and bone mineral density. Lancet 1992;340:9-13.

32. Kim CW, Hong S, Oh SH, et al. Change of bone mineral density and biochemical markers of bone turnover in patients on suppressive levothyroxine therapy for differentiated thyroid carcinoma. J Bone Metab 2015;22:135-141.



33. WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser 1994;843:1-129.

34. Leslie WD, Johansson H, Kanis JA, et al. Lumbar spine texture enhances 10-year fracture probability assessment. Osteoporos Int 2014;25:2271-2277.



35. Bousson V, Bergot C, Sutter B, et al. Trabecular bone score (TBS): available knowledge, clinical relevance, and future prospects. Osteoporos Int 2012;23:1489-1501.



36. Sampath TK, Simic P, Sendak R, et al. Thyroid-stimulating hormone restores bone volume, microarchitecture, and strength in aged ovariectomized rats. J Bone Miner Res 2007;22:849-859.


Fig. 1
Diagnosis of dual energy X-ray absorptiometry (DXA) during follow-up. For DXA, T-scores ≥−1.0 standard deviations (SDs) from the reference mean were defined as normal; T-scores between −2.5 and −1.0 SDs from the reference mean were defined as osteopenia; T-scores ≤−2.5 SDs from the reference mean were defined as osteoporosis. This follows the World Health Organization criteria. TSH, thyroid-stimulating hormone.
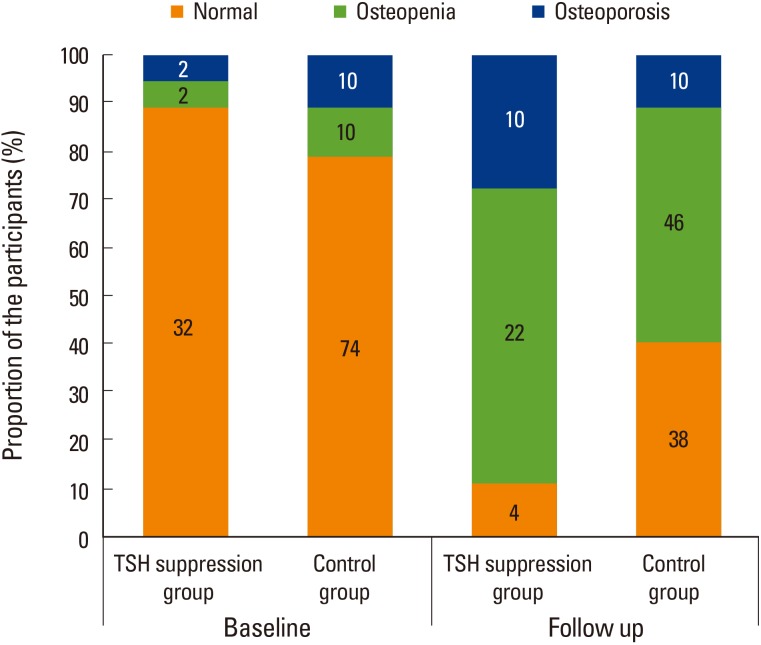
Fig. 2
Changes of mean areal bone mineral density (aBMD) and trabecular bone score (TBS) at the 1st dual energy X-ray absorptiometry scan and 2nd follow-up scan. Both (A) aBMD and (B) TBS did not change significantly during follow-up using paired t-test. TSH, thyroid-stimulating hormone.
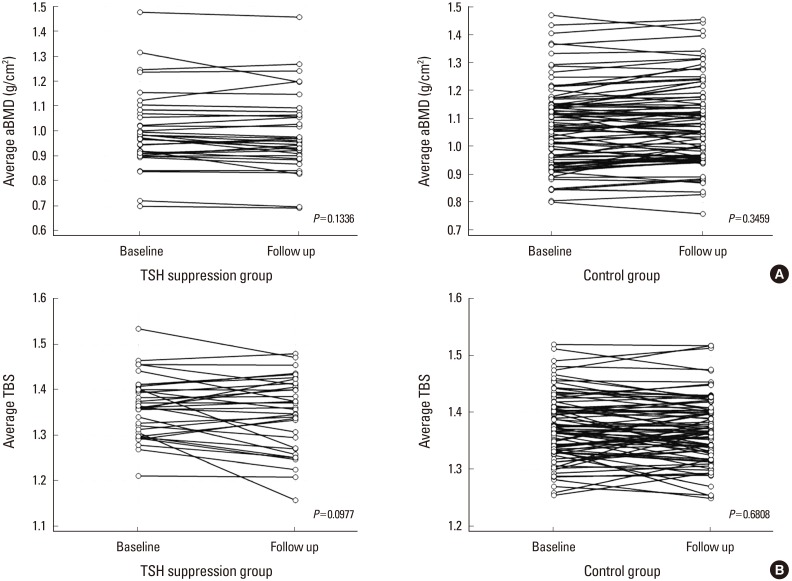
Table 2
Clinical and laboratory values during baseline and follow-up dual energy X-ray absorptiometry evaluations

The data is presented as the mean±standard deviation, or median (interquartile range).
a)P<0.01 vs. the control group.
F/U, follow-up; DXA, dual energy X-ray absorptiometry; BMI, body mass index; TSH, thyroid-stimulating hormone; Ca, calcium; ALP, alkaline phosphatase; BMD, bone mineral density; TBS, trabecular bone score.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print