α-Tocopheryl Succinate Inhibits Osteoclast Formation by Suppressing Receptor Activator of Nuclear Factor-kappaB Ligand (RANKL) Expression and Bone Resorption
Article information
Abstract
Objective
Osteoclasts are bone-resorbing multinucleated cells derived from the monocyte/macrophage lineage during normal and pathological bone turnover. Recently, several studies revealed that alpha-tocopheryl succinate (αTP-suc) have demonstrated potent anti-cancer activities in vitro and in vivo. However, the effects of αTP-suc on osteoclast formation and bone resorption remain unknown. Thus, in this study, we examined the effects of αTP-suc on osteoclast differentiation and bone resorbing activity in inflammatory bone loss model.
Methods
Osteoclast differentiation assay was performed by cocultures of mouse bone marrow cells and calvarial osteoblasts in culture media including interleukin-1 (IL-1). Osteoclasts were stained for tartrate-resistant acid phosphatase (TRAP). The level of receptor activator of nuclear factor-kappaB ligand (RANKL) mRNA was determined by reverse transcriptase-polymerase chain reaction (RT-PCR). ICR mice were administered an intraperitoneal injections of αTP-suc or dimethyl sulfoxide (DMSO) 1 day before the implantation of a freeze-dried collagen sponge loaded with phosphate-buffered saline (PBS) or IL-1 over the calvariae and every other day for 7 days. The whole calvariae were obtained and analyzed by micro-computed tomography (CT) scanning, and stained for TRAP.
Results
αTP-suc inhibits osteoclast formation in cocultures stimulated by IL-1 and decreased the level of expression of RANKL mRNA in osteoblasts. In addition, administered intraperitoneal injections of αTP-suc prevented IL-1-mediated osteoclast formation and bone loss in vivo.
Conclusion
Our findings suggest that αTP-suc may have therapeutic value for treating and preventing bone-resorptive diseases, such as osteoporosis.
INTRODUCTION
The bone tissue forms cartilages and the skeletal system, supports and adheres muscles with its mechanical functions, protects organic system and bone marrows and preserve them to maintain the homeostasis of calcium and phosphoric ions. The bone tissue consists of various cells including collagen, cytoplasmic matrix like glycoprotein, osteoblasts, osteoclasts and osteocytes. In addition, the bone tissue is continuously dissembled a little bit, generates new bones in proportion to the dissembled bones and is a very dynamic organ which maintains the homeostasis. The bone remodeling is maintained through proper balance between bone resorption by osteoclasts derived from the hematopoietic stem cell and bone formation by osteoblasts derived from the mesenchymal stem cell inside the marrow.[1,2] Overactive osteoclast causes imbalance the bone remodeling and metabolic bone diseases including periodontal disease by bacterial/germ infection, as well as osteoporosis, metastatic cancer by tumors, rheumatic arthritis and degenerative arthritis.[3,4] The receptor activator of nuclear factor-kappaB ligand (RANKL) is produced from the osteoblast by inflammatory cytokine like interleukin-1 (IL-1), becomes a crucial factor in creating and activating the osteoclast and plays a crucial role in differentiation from osteoclast precursors into mature osteoclasts under the existence of macrophage colony-stimulating factor (M-CSF).[5-7] Recently, Xiong et al.[8] reported that RANKL produced from osteocytes and chondrocytes in the substrate were essential for both osteoclast formation and its activation. In addition, the RANKL activates various signaling pathways by binding with RANKL expressed from the osteoclast precursors and is known as a requirement for differentiating and activating the osteoclasts.[3]
The structural formula of vitamin E was firstly reported in 1922 and is known as playing an important role in the reproduction process.[9] The vitamin E consists of tocopherol and tocotrienol depending on the existence of saturation in the site chain and has the types of alpha, beta, gamma and delta. The four types are separated depending on the numbers and locations of methyl groups in the chromanol ring structure.[10] The alpha (α)-tocopherol is abundant in human body and the vitamin E is the most representing substance. That is why the material has been under many researches but its biological studies have not been emerged. For example, the gamma (γ)-tocopherol decreases the cyclooxygenase-2 (COX-2) activation in the macrophages treated with the lipopolysaccharide (LPS) and epithelial cells treated with IL-1 and inhibits the creation of prostaglandin E2 (PGE2) but not much by α-tocopherol.[11] Aggarwal et al.[10] reported that the tocotrienols with different types increase anti-cancer and nerve cell protection effects but not by α-tocopherol. Meanwhile, Prasad and Edwards-Prasad[12] reported that the α-tocopheryl succinate (αTP-suc), one of esterified compound of the vitamin E was the most effective in anti-cancer activities than other vitamin E derivants including α-TP acetate and α-TP nicotinate. In addition, it was reported that cancer treatment was improved by using αTP-suc assistant while performing radiation and chemotherapy.[13] Recently, we found that α-tocotrienol strongly inhibited bone resorption and osteoclast formation by inhibiting the RANKL expression on the osteoblast but α-tocopherol had no such effect.[14] However, studies on the effects of αTP-suc in the aspect of generating and following bone reduction have been rare despite various advantages of αTP-suc mentioned above.
In this study, we seeks to discover how αTP-suc affected the osteoclast generation, the relation with the RANKL expression in the osteoblast known as an essential factor for the osteoclast generation and the effect of αTP-suc on the bone destruction stimulated by IL-1 in vivo.
MATERIALS AND METHODS
1. Material
Cell culture media and serum were purchased from Invitrogen (Carlsbad, CA, USA). The M-CSF, RANKL and IL-1 were purchased from PeproTech (London, UK) and 1α,25(OH)2D3, αTP-suc, α-tocopherol and αTP acetic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2. Osteoclast differentiation
The macrophages from the mouse bone marrow were obtained by the method mentioned in previous studies.[15] The macrophages were treated by the M-CSF (30 ng/mL) and the RANKL (100 ng/mL) for 4 days in the α-minimal essential medium (MEM) complete media which contained 10% (v/v) fetal bovine serum, 100 U/ml penicillin and 100 µg/mL streptomycin with the density of 4 × 104/well on the 48-well plate to induce the differentiation to the mature osteoclast. The complete media which contained the M-CSF and the RANKL were newly replaced after 3 days. The cells were stained with the tartrate-resistant acid phosphatase (TRAP) solution (Sigma-Aldrich) and the cells with more than 3 nucleus and positive to the staining were considered as the osteoclast after the cell cultures. The osteoclast differentiation induced by co-cultures of the bone marrow cells and the osteoblasts was performed based on the methods in previous studies.[15] In short, the M-CSF and the RANKL expressions were excited from the osteoblast by treating the IL-1 for 7 days with the density of 2 × 105/well of bone marrow cells and 2 × 104/well of the osteoblasts in the 48-well plate to induce the osteoclast formation, stained with the TRAP solution and the osteoclast was considered with more than 3 nucleus and positive to TRAP staining.
3. Enzyme linked immunosorbent
αTP-suc or the dimethyl sulfoxide (DMSO) was pretreated for 12 hours while culturing the osteoblast with the density of 3.5 × 105/well in the 6-well plate and the IL-1 or 1α,25(OH)2D3 was treated for 24 hours. The protein amount of the RANKL, osteoprotegerin (OPG) and PGE2 in the cell lysates or cell culture media was measured by performing enzyme immunoassay method based on the instruction of the manufacturer using the ELISA kit of R&D Systems (Minneapolis, MN, USA).
4. Reverse transcriptase-polymerase chain reaction (RT-PCR)
The RNA was prepared by using the TRIzol reagent (Invitrogen) based on the manufacturer's instruction and the cDNA was synthesized in the total RNA of 2 µg using the SuperScript II Preamplification System (Invitrogen). All the PCR amplifications were reacted by the Taq polymerase (Bioneer, Daejeon, Korea) and the primer sets used in the study were RANKL; 5'-CAGGTTTGCAGGACTCGAC-3' (sense), 5'-AGCAGGGAAGGGTTGGACA-3' (antisense), OPG; 5'-CCACTCTTATACGGACAGCT-3' (sense), 5'-TCTCGGCATTCACTTTGGTC-3' (antisense), Cox-2; 5'-TCAGCCAGGCAGCAAATCCT-3' (sense), 5'-TAGTCTCTCCTATGAGTATG-3' (antisense), M-CSF; 5'-CATCCAGGCAGAGACTGACA-3' (sense), 5'-CTTGCTGATCCTCCTTCCAG-3' (antisense), GAPDH; 5'-ACCACAGTCCATGCCATCAC-3' (sense), 5'-TCCACCACCCTGTTGCTGTA-3' (antisense). All PCR condition was performed by the degeneration for 30 seconds at the temperature of 94℃, annealing for 30 seconds at the temperature of 58 or 55℃ (M-CSF primer set), cycling for 20-30 times of expansion for 1 minute at the temperature of 72℃ and separating from the agarose gel which contained the Ethidium bromide. The expression amount was quantified by the Image-Pro Plus 4.0 (Media Cybernetics, Silver Spring, MD, USA).
5. IL-1-induced bone loss in vivo
All animal experiments were reviewed and approved by the Seoul National University School of Dentistry Animal Care Committee (Seoul, Korea). Lyophilized collagen sponge soaked with the IL-1 (2 µg) or the phosphate-buffered saline (PBS) was implanted to 5 mice in the test group and 5 mice in the control group (5-week old, male, ICR) on the calvarial bone to induce bone loss by IL-1.[16] The αTP-suc (80 mg/kg body weight) was intraperitoneally administered one day before implantation the collagen sponge and injected for 7 days for every 2 days after the operation. The same amount of αTP-suc, 30 µL of the DMSO solution, was intraperitoneally administered to the control group. All the mice were anesthetized 7 days after the operation, the calvarial bones were washed with the PBS and fixed with 4% of paraformaldehyde for one day. The calvaria were analyzed with the methods mentioned in previous studies to achieve 3-dimensional (3D) images (VGstudio MAX 1.2.1 program; Volume Graphics, Heidelberg, Germany) using high resolution micro-CT (SMX-90CT system; Shimadzu, Kyoto, Japan).[17] The 3D image files were used to measure the bone mineral content (BMC) through the TRI/3D-VIE (RATOC System Engineering, Kyoto, Japan) program. In addition, the whole calvaria was stained by the TRAP solution and the number of osteoclasts per unit cancellous bone surface dyed with red was measured with the manufacturer's instruction using the OsteoMeasure XP Version 1.01 (OsteoMetrics, Decatur, GA, USA) program.
6. Statistical analysis
ll the data were expressed as the average ± the standard deviation and the differences were analyzed by the Student's t-test for 2 groups and the one-way ANOVA (SPSS statistical software version 12; SPSS Inc., Chicago, IL, USA) for more than 3 groups. The data with less than 0.05 of P were expressed with asterisk (*) for significance.
RESULTS
1. The effect of αTP-suc on the osteoclast differentiation induced by co-culture of osteoblasts and bone marrow cells
To investigate the effect of the αTP-suc on the osteoclast differentiation, the osteoblasts and bone marrow cells isolated from mice were co-cultured in the presence of IL-1 to induce osteoclast formation. The result of checking the osteoclast differentiation through the TRAP staining after cell cultures for 7 days with different concentrations of αTP-suc showed that the group treated with the αTP-suc significantly inhibited osteoclast formation dependent on concentration compared to the control group (Fig. 1A, 1B). Such inhibition of osteoclast differentiation did not appear in the α-tocopherol and αTP acetate (Fig. 1B). As such, the αTP-suc may strongly inhibit the osteoclast differentiation induced by co-culture unlike different αTPs.
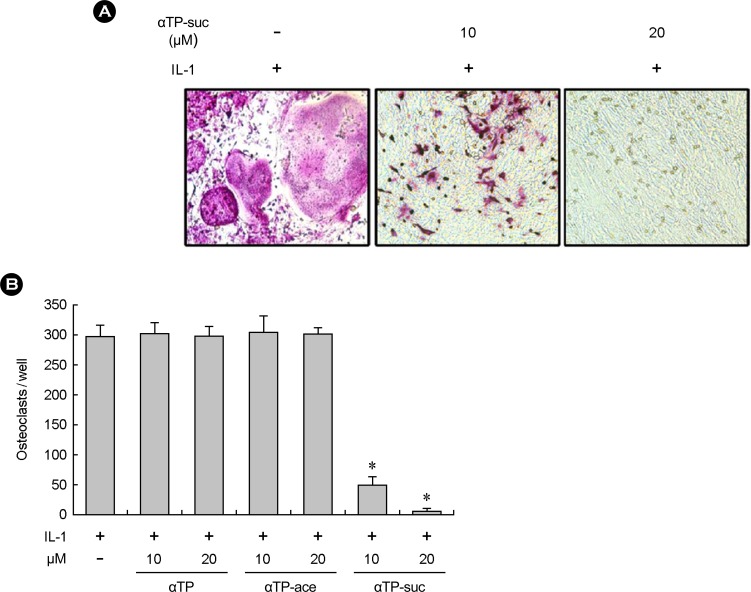
Effects of alpha-tocopheryl succinate (αTP-suc) on osteoclast differentiation in coculture. (A) Tartrate-resistant acid phosphatase (TRAP) staining of cocultures in the presence of interleukin-1 (IL-1; 15 ng/mL) for 7 days with or without αTP-suc (10 µM or 20 µM). (B) Bone marrow cells and calvarial osteoblasts were cultured with IL-1 (15 ng/mL) in the presence or absence of αTP-suc, αTP acetate (ace) or αTP. After 7 days, cells were fixed and stained for TRAP. TRAP-positive multinucleated cells containing three or more nuclei were counted as osteoclasts. *P < 0.05 versus dimethyl sulfoxide-treated control.
2. The effect of the αTP-suc on the RANKL-induced osteoclast formation in bone marrow-derived macrophages
Next, the macrophages from the bone marrow were cultured for 4 days by stimulating the αTP-suc with different concentrations under the existence of the M-CSF and the RANKL as the osteoclast precursors to investigate direct inhibition from the osteoclast differentiation of the αTP-suc for the osteoclast precursors obtained from the mouse bone marrow cells. The result of checking the osteoclast differentiation through the TRAP staining showed that TRAP-positive osteoclast had no changes of amount similar to α-tocopherol in case of treating αTP-suc with different concentrations (Fig. 2A, 2B). The results mean that αTP-suc did not directly affect the osteoclast precursors and the substance may inhibit the osteoclast differentiation by affecting the osteoblast.

Effects of alpha-tocopheryl succinate (αTP-suc) on receptor activator of nuclear factor-kappaB ligand (RANKL)-induced osteoclast formation in bone marrow-derived macrophages. (A) Tartrate-resistant acid phosphatase (TRAP) staining of osteoclast precursor cultures in the presence of 30 ng/mL macrophage colony-stimulating factor (M-CSF) and 100 ng/mL RANKL for 4 days with or without αTP-suc (20 µM) or αTP (20 µM). (B) Bone marrow-derived macrophages were cultured with the indicated doses of αTP-suc, αTP-ace or αTP in the presence of M-CSF and RANKL. After 4 days, TRAP-positive multinucleated cells containing three or more nuclei were counted as osteoclasts.
3. The effect of αTP-suc on the RANKL expression in the osteoblasts
The RANKL is a member of tumour necrosis factor (TNF) family, crucial for the osteoclast differentiation and known to be expressed from the osteoblast by stimulation from factors in stimulating the osteoclast differentiation including 1α,25(OH)2D3, PGE2 and IL-1. Therefore, we performed the RT-PCR and the enzyme immunoassay to investigate the effect of αTP-suc on the RANKL expression in the osteoblasts. The result of treating the IL-1 and 1α,25(OH)2D3 in the osteoblast shows that the mRNA and protein expressions increased in the RANKL and the mRNA and protein expressions in the OPG known as the decoy receptor antagonized against the RANKL decreased (Fig. 3A and B). The RANKL expression increased by stimulating factors of the osteoclast differentiation was strongly inhibited by the αTP-suc treatment but did not much affect the OPG expression (Fig. 3A, 3B). As well, αTP-suc did not affect the expression and generation of M-CSF, COX-2 and PGE2. These results show that the inhibition of the osteoclast differentiation by αTP-suc was directly related to inhibiting the RANKL expression in the osteoblast.
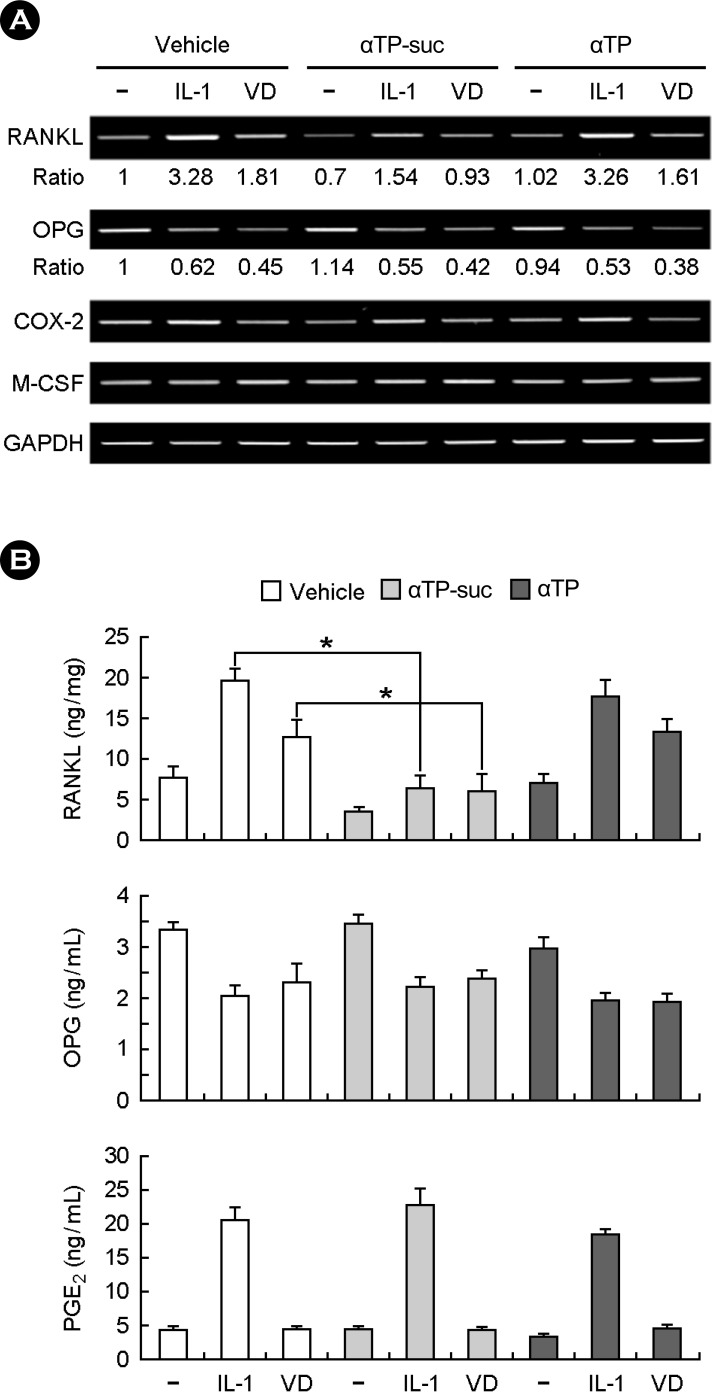
Effects of alpha-tocopheryl succinate (αTP-suc) on interleukin-1 (IL-1)-induced receptor activator of nuclear factor-kappaB ligand (RANKL) expression in osteoblasts. (A and B) Mouse calvarial osteoblasts were pretreated with αTP-suc (20 µM), αTP (20 µM) or dimethyl sulfoxide (DMSO) for 12 h and then cultured with IL-1 (15 ng/mL) or 1α,25(OH)2D3 (VD; 10 nM) for 24 h. (A) The mRNA levels of the indicated genes were analyzed by reverse transcriptase-polymerase chain reaction (RT-PCR). (B) The indicated protein amounts were determined by using enzyme Immunoassay (ELISA) kits in cell lysates (RANKL) and in cell culture media (osteoprotegerin and prostaglandin E2). *P < 0.05.
4. The effect of αTP-suc on IL-1-induced bone destruction in vivo
It was confirmed that the αTP-suc inhibited the osteoclast formation in vitro studies. Next, we investigated the effect of αTP-suc on IL-1-induced bone destruction in vivo. The IL-1 is a strong cytokine related to inflammation and it was shown that treating the IL-1 lead to severe calvarial bone loss through the TRAP satining (Fig. 4A, top) and micro-CT images (Fig. 4A, bottom) but the mice injected with αTP-suc showed strong inhibition of bone losses due to the IL-1 (Fig. 4A). In addition, the BMC lost by the IL-1 was significantly restored by αTP-suc (Fig. 4B) and the number of TRAP-positive osteoclasts per unit area increased by the IL-1 was significantly decreased by αTP-suc (Fig. 4B). These results show that αTP-suc inhibited the osteoclast formation and its following bone losses in vivo like at the cell level.

Effects of alpha-tocopheryl succinate (αTP-suc) on IL-1-induced bone destruction in vivo. (A-C) A collagen sponge treated with PBS or IL-1 (2 µg) was implanted over the calvarial bone of 5-week-old mice. αTP-suc (80 mg/kg body weight) or vehicle (DMSO) was administered intraperitoneally for 7 days. (A) Whole calvariae were fixed and stained for TRAP to identify eroded surfaces (top), and presented as 3D micro-CT images (bottom). (B) Calvarial bone mineral content was measured by micro-CT analysis. (C) Osteoclast number was analyzed by histomorphometric analysis. *P < 0.05 versus control group; #P < 0.05 versus group treated with IL-1 only.
DISCUSSION
The effect of αTP-suc on the bone biology, especially on the osteoclast differentiation and bone loss is rarely known even though it is reported that αTP-suc was the most effective in anti-cancer activities than other vitamin E derivants and very effective as the adjuvant to the cancer treatment.[13] Prior to the study, it was reported that α-tocotrienol, one of vitamin E series, inhibited the osteoclast generation and bone resorption.[14] The α-tocotrienol strongly inhibited the RANKL expression in the osteoblast, known as a critical factor in the osteoclast differentiation and the c-Fos expression in osteoclast precursors. In addition, inhibiting differentiation to mature osteoclasts from the osteoclast precursors by α-tocotrienol was completely restored by the excessive expression of the c-Fos. In addition, it was found out that α-tocotrienol strongly inhibited the bone resorption by the mature osteoclasts through the calcium-phosphate apatite-coated OAAS plate.[14] However, the other vitamin E series, α-tocopherol, did not show the osteoclast differentiation and the bone resorption.[14] Other study groups reported that the vitamin E was required for bone calcification and reformation and it was found out that α-tocotrienol was more effective in bone protection than α-tocopherol.[18-21] Meanwhile, it was interesting that Fujita et al.[22] reported that the genetic mouse model of vitamin E by eliminating the α-tocopherol transfer protein showed increase in bone mass by decreasing bone resorption and the α-tocopherol treatment at the cell level improved the osteoclast fusion. The differences in results suggest that the causes are due to methods for each experiment and ages of mice, meaning that additional studies are required to investigate the relation between the vitamin E and the bone metabolism.
This study discovered that αTP-suc, one of the esterified compound of the vitamin E, strongly inhibited the osteoclast differentiation and bone losses unlike the αTP acetate and α-tocopherol, another derivants of the vitamin E. First, we treated αTP-suc in the osteoclast precursors with different concentrations under the existence of the M-CSF and the RANKL to investigate how the osteoclast differentiation strongly inhibited by αTP-suc was inhibited in the co-culture of the osteoblasts and the bone marrow cells. However, we found that the αTP-suc did not affect the osteoclast formation by the RANKL in such systems. It may be suggested that the results inhibited the osteoclast differentiation by affecting the osteoblast which induced the osteoclast differentiation, rather than the fact that αTP-suc did not directly affect the osteoclast precursors.
Then, the effect of αTP-suc for the RANKL expression in the osteoblast was investigated. It was known that the RANKL was required for the osteoclast differentiation and various stimulating factors in the osteoclast differentiation including IL-1, TNF-α and 1α,25(OH)2D3 increased the RANKL expression and induced the osteoclast differentiation.[4] In particular, IL-1 is considered as one of the most important mediator in the osteoporosis accompanying chronic bone resorption by estrogen deficiency or various inflammatory factors.[23,24] Lorenzo et al.[24] reported that the mice with deficient IL-1 receptors did not show decrease in the bone mass due to the ovariectomy and Abramson and Amin[25] discovered that blocking the IL-1 signal system decreased bone destruction and cartilage losses in the animal model with rheumatic arthritis. Interestingly, the αTP-suc strongly inhibited the RANKL expression increased by the IL-1 in the osteoblasts (Fig. 3). The IL-1 stimulated the PGE2 generation and increased the RANKL expression and it was known that the PGE2 generation was performed by the cytosolic phospholipase A2 (cPLA2) and the COX-2.[16,26] In addition, it was reported that some vitamin E may inhibit the PGE2 generation in the cells. For example, α-tocotrienol inhibited the cPLA2 activation induced by the glutamate in the HT4 nerve cells[27] and long-chain carboxychromanols of vitamin E impeded the COX-2 activation in the A549 lung epithelial cells and decreased the PGE2 production induced by the IL-1.[28] However, we found that the mRNA expression of COX-2 and protein production of PGE2 induced by the IL-1 was not affected by the αTP-suc (Fig. 3). In addition, the osteoclast differentiation by the αTP-suc and the RANKL expression inhibition were not restored by directly stimulating the PGE2 into the cells (not published). Therefore, the results suggest that the αTP-suc affected PGE2 subsequent factors or other new factors in the osteoblast and may decrease the RANKL expression. In addition, the Western blotting confirmed that the αTP-suc affected nothing on the activation of NF-κB signaling pathways and the mitogen activated protein kinases (MAPKs) including the extracellular signal-regulated kinase (ERK), c-JUN N-terminal kinase (JNK) and p38 induced by the IL-1 in the osteoblast (not published). Therefore, it is considered that there shall be studies on specific routes of the RANKL expression inhibition from the αTP-suc in the osteoblast. One of potential mechanisms which may be proposed to the RANKL expression inhibition by the αTP-suc is that the αTP-suc may inhibit the RANKL promoter activation, as well as the mRNA expression. It was well-reported that the vitamin D stimulated response elements on the RANKL promoters in the osteoblasts of the human and mice and induced the RANKL expression, stimulating the osteoclast differentiation[29,30] even though it was not well-known that the vitamin E may adjust the activation of the RANKL promoters. In addition, Staal et al.[31] reported that the transforming growth factor β (TGF-β) inhibited the stimulation of the vitamin D response elements in the osteoblast and transcription and expression of the target transcription factors. Additionally, the present study showed that the RANKL expression increased by the vitamin D was inhibited by the αTP-suc (Fig. 3). Therefore, additional studies are required to discover whether the activation of the RANKL promoters induced by the IL-1 or the vitamin D is inhibited by the αTP-suc.
Lastly, we investigated whether the inhibition of osteoclast differentiation by αTP-suc in vitro was reproduced in the organism by using the mouse model. Injecting the αTP-suc strongly inhibited the excessive bone losses and the osteoclast formation on calvarial bone induced by IL-1. The results coincide with the study results in vitro and may suggest the possibility that the RANKL expression may be inhibited in the osteoblast by the αTP-suc in vivo. However, additional studies are required to investigate whether the RANKL expression in vivo is inhibited by the αTP-suc. In addition, it was reported that the RANKL produced from the chondrocytes and ostecytes were required to form and activate the osteoclasts,[8] meaning that the effect of the αTP-suc on the RANKL expression from these cells shall be investigated.
CONCLUSION
The αTP-suc inhibited the osteoclast formation in the co-culture system which consisted of the osteoblasts and the bone marrow cells but failed to inhibit the differentiation of the osteoclast precursors to mature osteoclasts by the RANKL. Also, the αTP-suc decreased the RANKL expression in the osteoblasts. This study confirmed the osteoclast formation and bone destruction inhibition by the αTP-suc in vivo using the mouse model. Therefore, this study results indicated that the αTP-suc may be efficiently utilized to prevent and treat metabolic bone diseases including the osteoporosis due to excessive bone losses.
Notes
This work was supported by the National Research Foundation of Korea (NRF, Grant 2010-0015131) and a Science Research Center grant through the Bone Metabolism Research Center funded by the Korea government (MEST, Grant 2011-0001023).