|
 |
| jbm > Volume 22(1); 2015 > Article |
|
Abstract
Wilson's disease is a rare genetic disorder that has abnormal copper metabolism. Although the disease's main problems are found in liver and brain, some studies revealed manifestation of various musculoskeletal problems in the patients. In this report, we encountered a young patient who had fracture in the forearm bone. Initially, exception to a previous history of fracture from a motorcycle accident, the patient did not have any medical or drug use history, and laboratory work-ups were insignificant. However, with suspicion on his bone's integrity, bone densitometry was recommended and revealed osteopenic change. To disclose a cause for the change, questions were made to recall any particular history or event, and his complaint of recent vision loss led to ophthalmologic consultation where under slit-lamp test found Kayser-Fleischer ring. Further laboratory work-up found low levels of serum copper and ceruloplasmin and high copper level in 24-hr urine sample that led to the diagnosis of Wilson's disease. Although Wilson's disease has been frequently noticed with considerable musculoskeletal manifestation, it rarity makes the diagnosis illusive to a physician. Hence, despite of its rarity, it is imperative to remember the disease's bony manifestation, and it should be suspected in young patients with demineralized bone when the reason for brittle bone cannot be answered with other better known conditions.
Wilson's disease has abnormal accumulation of copper with characteristic manifestations in hepatic and neurologic systems.[1,2,3] It is a rare autosomal recessive disorder with mutation in the ATP7B gene on chromosome 13, and the prevalence is reported to be 30 per 1 million worldwide.[1,2] Although the disorder is manifested mainly in hepatic and neurologic systems, skeletal involvement has been discussed in several literatures.[1,4,5] They noticed diverse findings of bony involvements, and demineralization is one of commonly observed problems in the patients.[1,3,6] Common neurologic manifestations include tremor, dystonia and impairment of cognition and attention, and imbalance and incoordination are also usual dysfunctions found in the patients. These conditions make the patients to experience greater frequency of slip downs. Together with decreased bone mass, patients with the disease are thought to be associated with higher risk of fracture. In this case report, we experienced a young male patient with fracture in the forearm bones from a minor trauma. At first, exception to current presentation of the forearm fracture no findings were remarkable to make a suspicion of other clinical conditions. However, his history of multiple fractures made us to initiate a further study on bone quality, and this additional investigation had led us to make the diagnosis of Wilson's disease in the end.
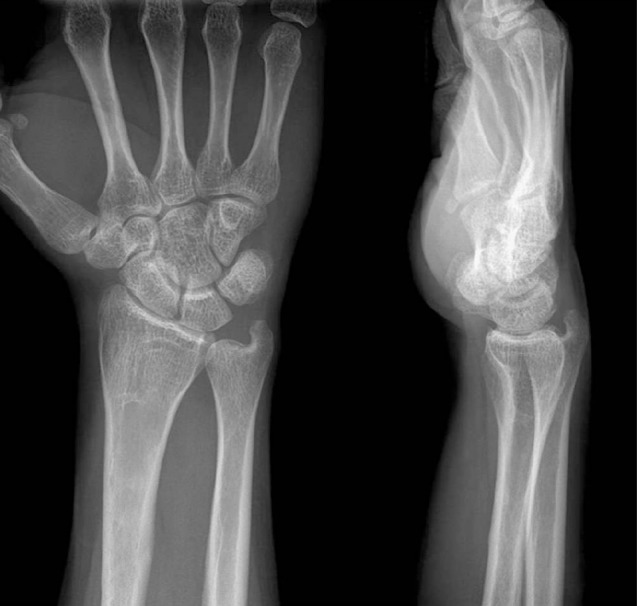
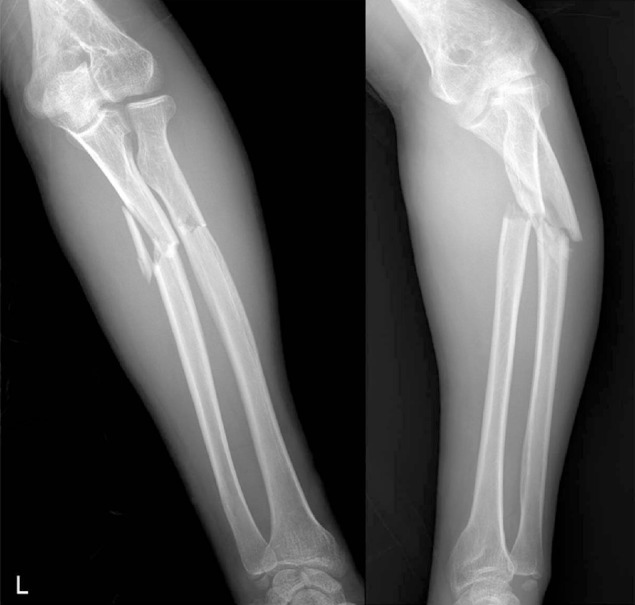
A 25-year-old male was admitted to our emergency department due to painful swelling in his left forearm. He experienced a slip down injury while walking. Simple radiograph revealed both radius and ulna fractures in proximal shaft (Fig. 1). His medical history was unremarkable. He had a history of motorcycle trauma which led to fractures to distal shaft of right radius and left distal humerus 5 years ago. He underwent an operation for open reduction and internal fixation on both fracture sites at our institution, and they healed without any complication. Second operation was performed to remove plates and screws 15 months after the surgery, and postoperative recovery was uneventful. Initial laboratory examinations revealed no abnormal findings. To treat his ulnar fracture, open reduction and internal fixation was chosen, and radial fracture was stabilized with a Khai nail under closed reduction. Postoperative radiography showed stable fixation for both bones (Fig. 2).
Postoperative recovery progressed without a problem. However, postoperative review of the radiographs raised a suspicion of mild demineralization (Fig. 3). In addition, the minor traumatic nature of his slip down accident coupled with a fracture history led to a concern about fragility of his bone. We recommended bone densitometry to evaluate his bone quality, and the examination disclosed osteopenia in the spine (Z score=-2.3) and femoral neck (Z score =-1.3). To find the cause of his osteopenic condition, further laboratory evaluations were made, and his medical and personal histories were questioned and reviewed.
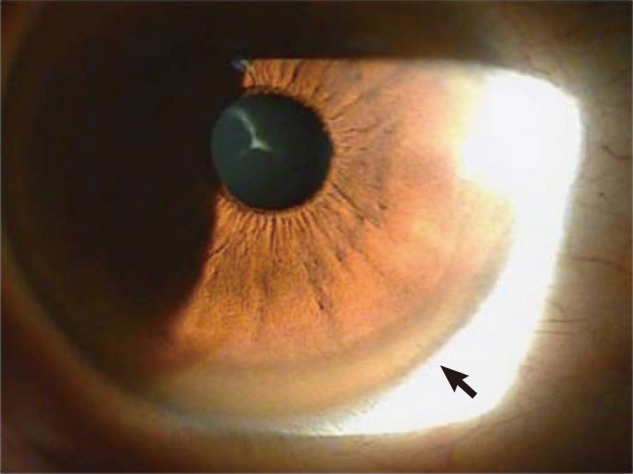
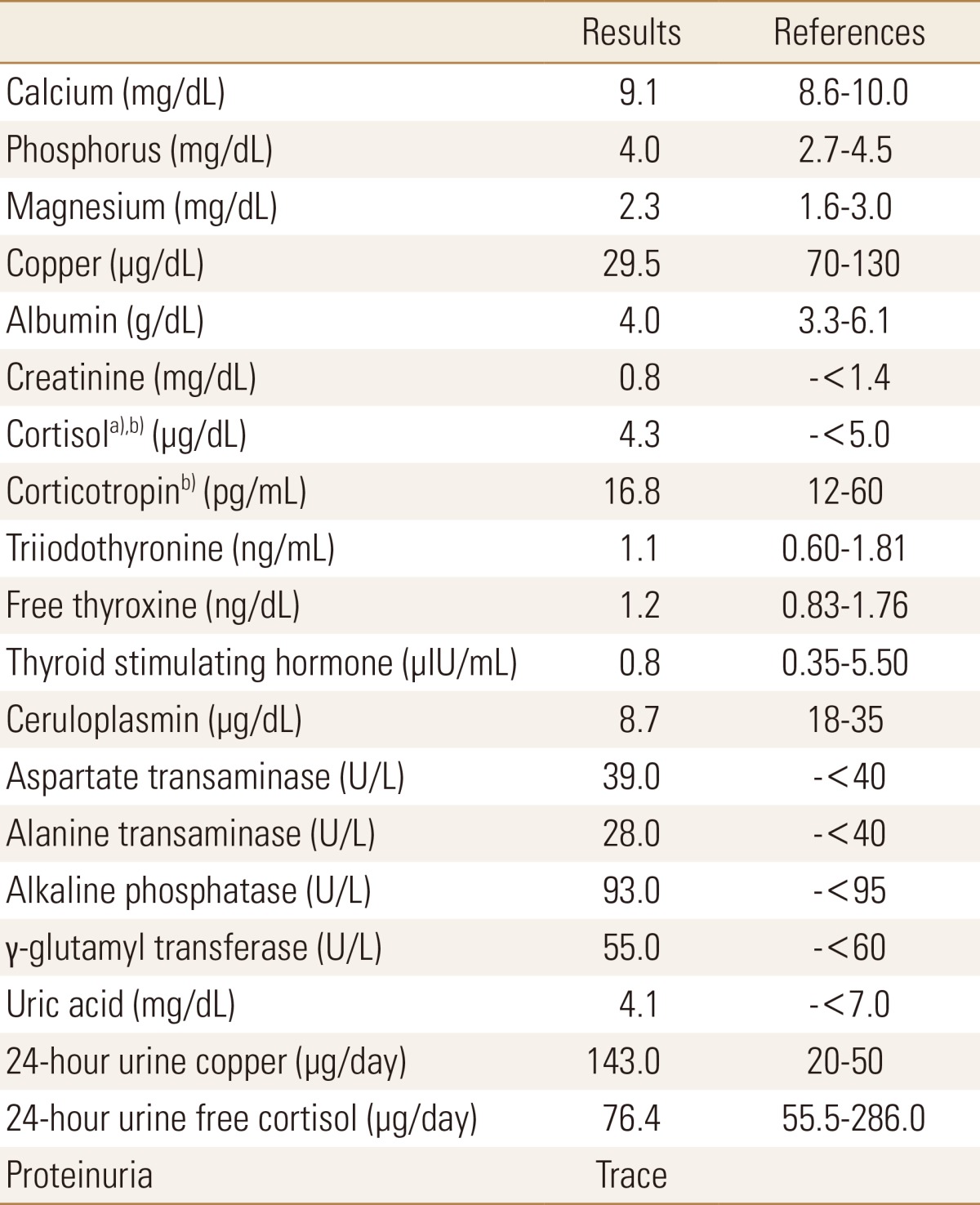
Initial laboratory examinations showed slight increase only in serum liver transferases. Nonetheless the results were insignificant to suspect a condition such as hyperthyroidism, Cushing syndrome, myeloma or osteomalacia that is associated with decreased bone mass. The patient did not have smoking, alcoholic or drug use history, and the review of his past medical history was insignificant. However, during an interview with his family his sister commented about the patient's slow performance as a child, and recently she had noticed progressively more sluggish and slower behavior of the patient. When further questions were made, the patient stated that his speech became unclear to the point where his coworkers often complained of difficulty in communication, and he had experienced many incidences of accidental slip downs and dropping an object and had felt uneasiness in maintaining a control of his body. In addition, he noticed blurring in his vision often though he had thought that the problem was from fatigue due to his job as a manual laborer. The patient was referred to the ophthalmology department, and brownish pigmentation in the cornea was observed under slit lamp examination (Fig. 4). Additional laboratory tests revealed decreased level of serum copper and ceruloplasmin, and copper level of 24-hr collected urine was elevated significantly from normal range (Table 1). These findings led to the diagnosis of Wilson's disease.
Wilson's disease is a rare genetic disorder that is mainly characterized by hepatic and neurologic manifestations.[1,2,3] In addition, several authors have noted its association with musculoskeletal impairments, and among the various impairments described, demineralization is found to be the most principle involvement (Table 2).[1,3,6] This problem may result in extraordinarily high rate of fracture even from a minor trauma that is not considered sufficient to fracture a bone. Additionally, the disease's neurologic consequence makes the patients more vulnerable to traumas, and combined with brittle bone the disorder is considered this particular patient group at higher risk of an orthopedic injury.
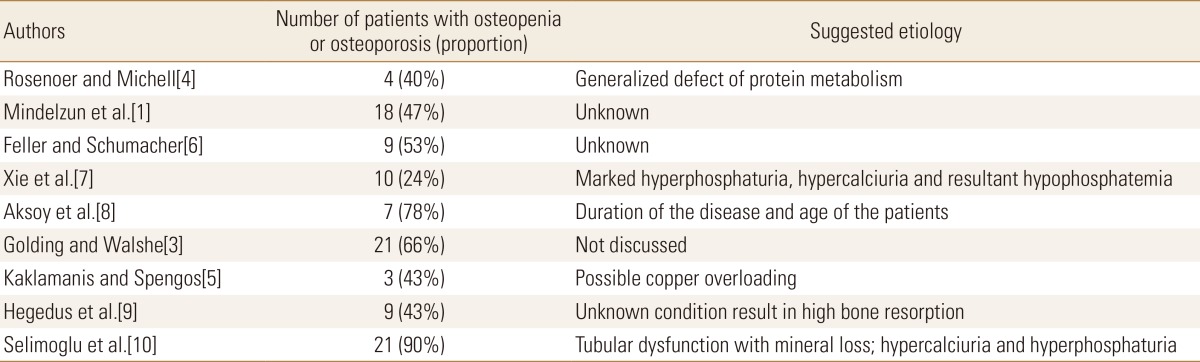
Previous studies showed significant proportions of their patients with bone loss from 24[7] to 78%.[8] However, their measurements are from observations of simple radiograph, and the subjective examination is considered imprecise to some extent in order to find the presence of demineralization. Use of bone densitometry is currently a generalized and reliable method to measure bone mineral density quantitatively. In their studies that utilized the densitometry, Hegedus et al.[9] and Selimoglu et al.[10] found osteoporosis in 43% and 67.7% of their study populations, respectively. In the latter study, osteopenia was also observed in 22.6%, and this makes the overall proportion of the patients with decreased bone quality to 90.3%.
Prevalence of traumatic fracture ranged between 7[7] and 16%[8] in literatures, and all the cases healed without a complication. In our case, the patient already had suffered fractures in his radial and humeral bones, and the bones healed without any complication. For the current injury, his follow up radiographs showed gradual union of bone. After 5 months of the surgery, no complication has been observed.
Although the high prevalent bone loss is evident, the cause for the loss in Wilson's disease is still under obscurity.[5,7] Renal tubular disorder with hypercalciuria, hepatic dysfunction, direct copper toxicity and factors including climate, nutritional intake, body mass index and immobilization are suggested as contributing causes for bone loss. In an animal study, dogs with deficient copper showed abnormal mineralization of bone,[4] and a vitro study involving chicken embryo found reduction in bone formation and disruption in osteoblast cycle.[2]
A recent study by Hegedus et al.[9] where serum bone metabolism markers in patients with Wilson's disease were measured found significantly increased levels of β-cross-laps, a marker for bone resorption activity, and osteoprotegrin, a product secreted by osteoblasts. They suggested that β-cross-laps level is elevated due to high bone resorption in Wilson's disease, resulting in compensatory activation of osteoblast along with high level of osteoprotegrin. Although the underlying cause is unknown, they confirmed presence of considerable bone resorptive state in Wilson's disease.
In conclusion, studies revealed highly prevalent osteoporotic change and elevated state of bone resorption in Wilson's disease. Together with neurologic consequences of Wilson's disease, the patients are exposed to more traumatic events that raise the risk of bone pathology. Thus, providing information on bony involvement of the disease, examining bone density in these patients and giving proper therapies for the associated bone pathologies are necessary to prevent or at least minimize the injury risk, and in young patients with bone demineralization whose cause for the change is under obscurity Wilson's disease should also be suspected. Further studies are required to disclose a principle etiology on highly resorptive state of bone evident in the disease.
References
1. Mindelzun R, Elkin M, Scheinberg IH, et al. Skeletal changes in Wilson's disease. A radiological study. Radiology 1970;94:127-132.


2. Quemeneur AS, Trocello JM, Ea HK, et al. Miscellaneous non-inflammatory musculoskeletal conditions. Musculoskeletal conditions associated with Wilson's disease. Best Pract Res Clin Rheumatol 2011;25:627-636.


3. Golding DN, Walshe JM. Arthropathy of Wilson's disease. Study of clinical and radiological features in 32 patients. Ann Rheum Dis 1977;36:99-111.



4. Rosenoer VM, Michell RC. Skeletal changes in Wilson's disease (hepato-lenticular degeneration). Br J Radiol 1959;32:805-809.


5. Kaklamanis P, Spengos M. Osteoarticular changes and synovial biopsy findings in Wilson's disease. Ann Rheum Dis 1973;32:422-427.



6. Feller ER, Schumacher HR. Osteoarticular changes in Wilson's disease. Arthritis Rheum 1972;15:259-266.


7. Xie YZ, Zhang XZ, Xu XH, et al. Radiologic study of 42 cases of Wilson disease. Skeletal Radiol 1985;13:114-119.



8. Aksoy M, Camli N, Dincol K, et al. Osseous changes in Wilson's disease. A radiologic study of nine patients. Radiology 1972;102:505-509.


Fig. 2
Immediate postoperative radiography showing fixation with a plate for ulna and a Khai nail for radius.

Fig. 3
Reexamination of the patient's initial radiograms raised suspicion of osteoarticular changes, and in this figure, distal portion of the radius has thinning of cortex, abnormal striation of trabeculae and sclerotic joint margin.
- TOOLS
-
METRICS

-
- 5 Crossref
- 0
- 4,638 View
- 15 Download
- Related articles
-
Hypertrophic Osteoarthropathy in Patient with Crohn's Disease: A Case Report2014 May;21(2)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print