Black Bone Disease: Ochronotic Arthritis Detected during Knee Arthroplasty
Article information
Abstract
Alkaptonuria is an extremely rare autosomal recessive metabolic disorder characterized by dark urine, ochronosis, and arthritis of the spine and major joints. We report a case of ochronotic arthritis observed during total knee replacement surgery in a 65-year-old male patient with no relevant medical history. Based on a literature review, this is the first case of ochronotic arthritis reported in Korea.
INTRODUCTION
Alkaptonuria is an extremely rare genetic disorder with an autosomal recessive inheritance and an incidence of 1 in 250,000 to 1,000,000 individuals.[1–4] The main features of the condition include dark urine, ochronosis, and arthritis of the spine and major joints.[5] Although there are many reported cases of alkaptonuria, cases in Korea are rare. There have been case reports of discolouration of the urine and rupture of the black crescent-shaped cartilage.[6–8] However, to date, there have been no reports of arthritis in alkaptonuria in Korea. Herein, we report a case of ochronotic arthritis observed during total knee arthroplasty with a literature review.
CASE REPORT
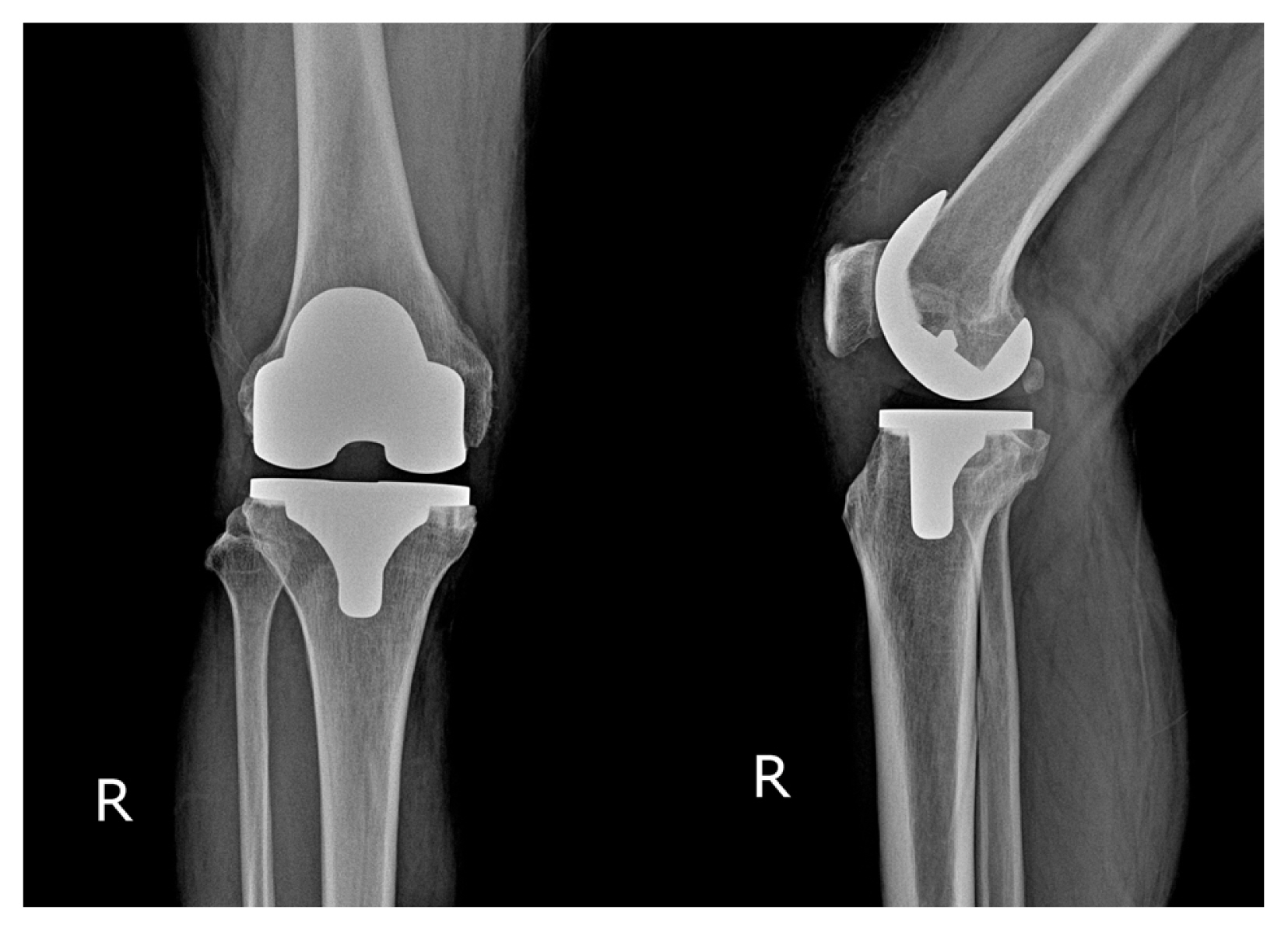
A 65-year-old male patient presented to our hospital with pain in the right knee that had persisted for several years. He was an outdoor labourer with no history of trauma or regular consumption of any medications. The patient did not complain of pain in any joint other than the right knee. On physical examination, the range of motion of the right knee joint was normal with no swelling or warmth. However, bluish-black pigmentation was observed on the legs and face (Fig. 1). The preoperative blood tests, including C-reactive protein, erythrocyte sedimentation rate, rheumatoid factor, and uric acid, showed normal results, and no abnormal findings were identified on urinalysis. On plain radiography, advanced osteoarthritis of the medial compartment of the right knee was observed (Kellgren–Lawrence grade 4); however, no abnormal findings were discovered in the surrounding bone tissue, such as signal changes on magnetic resonance imaging, except for signs of arthritis. On radiography of other areas, degenerative changes were observed in the lower thoracic spine; however, the hip and ankle joints were normal (Fig. 2A, B).

Arthritic changes in the right knee as observed on the pre-operative radiograph (A) and T2-weighted magnetic resonance image (B).
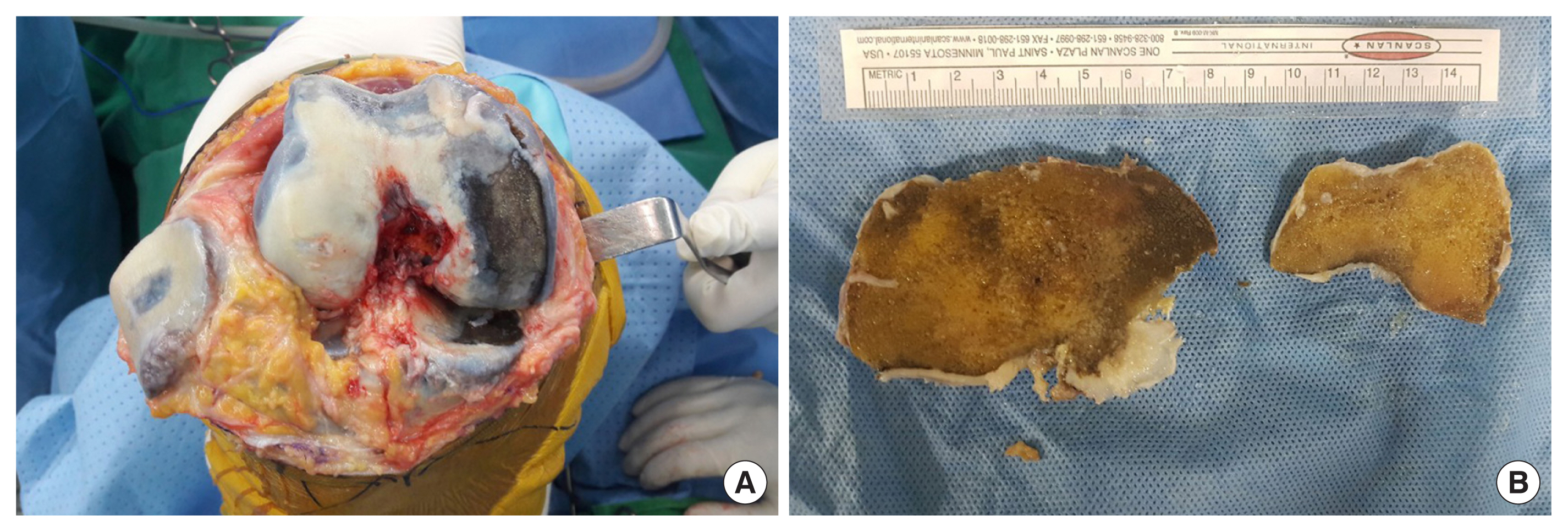
During total knee arthroplasty, black bones were observed in the subchondral and cancellous bones of the femur, tibia, and patella (Fig. 3A, B). No bacterial growth was detected in the cultures obtained during surgery. Moreover, after the surgery, satisfactory results were obtained during the recovery and rehabilitation processes (Fig. 4).
Clinically, ochronotic arthritis was not considered preoperatively, and the diagnosis of alkaptonuria was only made after the discovery of black bones during surgery. As the patient had no history of drug use that could have induced tissue ochronosis, exogenous tissue ochronosis was ruled out. The patient denied any family history of alkaptonuria. Furthermore, because of patient refusal, tests for urine discolouration, homogentisic acid (HGA) measurements, and genetic testing, which could have helped diagnose alkaptonuria, were not performed.
DISCUSSION
Ochronosis is a phenomenon first described by Virchow in 1866 in which specific tissues turn brownish-yellow.[2,9] It is classified into 2 types: exogenous ochronosis and endogenous ochronosis. Exogenous ochronosis is mainly caused by using drugs, such as antimalarials and tetracyclines and those containing hydroquinone and phenol.[10,11] Endogenous ochronosis is a systemic metabolic disorder caused by the deficiency of homogentisate 1,2-dioxygenase (HGO), which results in the accumulation of HGA in the body. HGA is an intermediate metabolite produced during the metabolism of the amino acids tyrosine and phenylalanine, which are oxidized to form benzoquinone acetic acid. During oxidation, free radicals are formed that cause tissue damage, and benzoquinone acetic acid inhibits lysyl hydroxylase, an enzyme that regulates collagen fibers and reduces their cross-linking.[2] Benzoquinone acetic acid is then oxidized again, forming a melanin-like pigment that darkens the tissues (Fig. 5). The pigment mainly accumulates in the sclera, nails, skin, bones, thyroid glands, coronary arteries, endocardium, and heart valves.[12] As HGA accumulates in the connective tissues, not only do the tissues change their colour but their structural integrity also weakens, resulting in inflammation and tissue break down.[3]
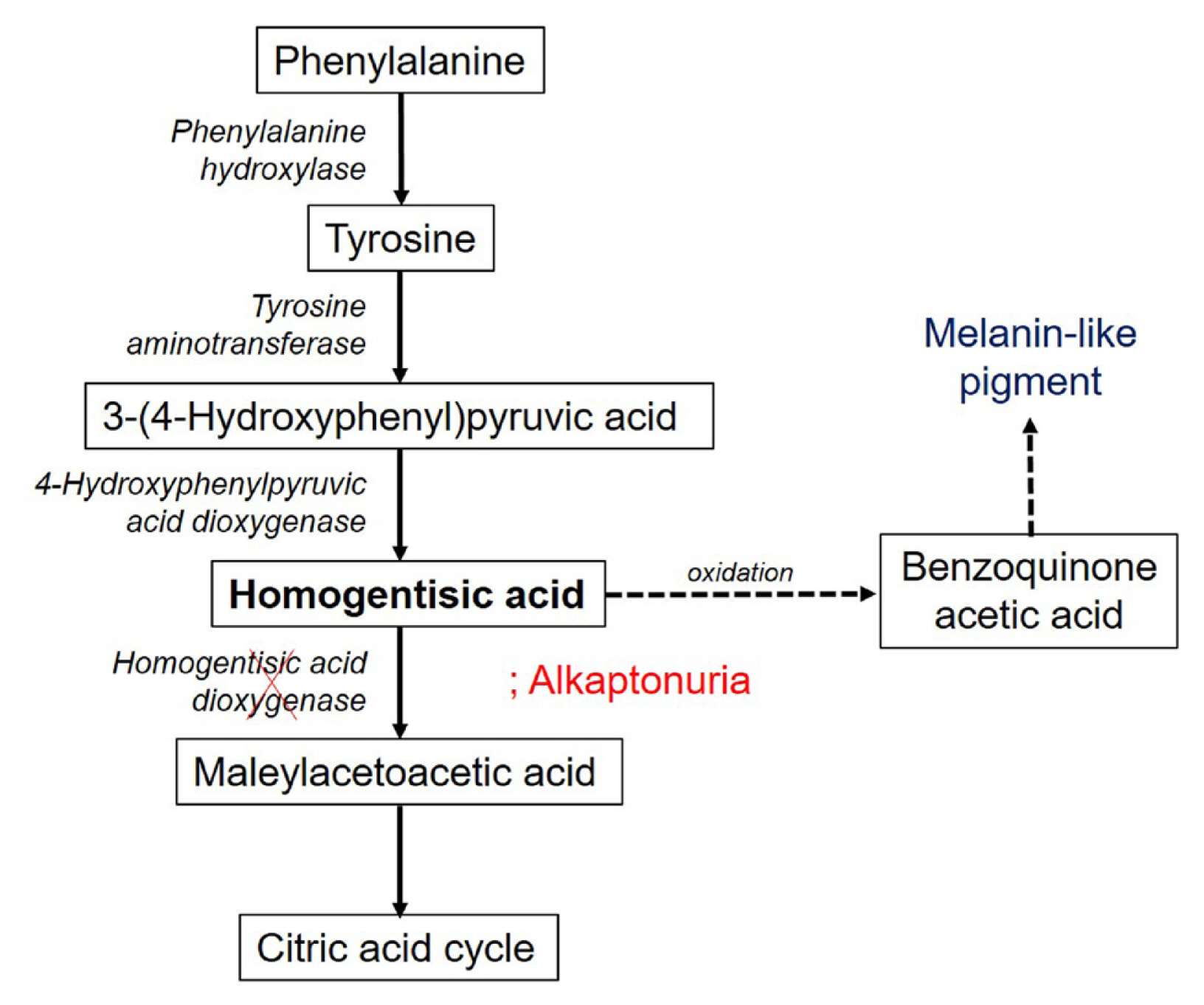
In alkaptonuria, mutations in the gene coding for homogentisic acid dioxygenase cause accumulation of homogentisic acid, leading to pigmentation in the connective tissue.
Alkaptonuria is often diagnosed at the paediatric age because of the darkening urine, even if no other symptoms are present. The urine of an individual with alkaptonuria is initially normal in appearance, but if left standing, it darkens gradually owing to exposure to air. After the third decade of life, affected individuals experience changes in skin colour and arthritis due to the long-term accumulation of HGA. In addition to arthritis, minor trauma to the musculoskeletal system along with tendon rupture has been reported, including Achilles tendon, patellar tendon, and quadriceps and hamstring ruptures.[13,14] Tendon rupture can be treated with a primary suture.[15]
There are no established diagnostic criteria for alkaptonuria; however, it is generally diagnosed based on clinical symptoms, and an increase in HGA levels observed on urinalysis and serum can aid in diagnosis.[16] Recently, mutations in the HGO gene on chromosome 3q have been confirmed by molecular diagnosis.[5] In this case, the patient was clinically diagnosed with alkaptonuria owing to bluish-black pigmentation of the skin, arthritis of the spine and knee, and black bones. Exogenous ochronosis was excluded from the diagnosis, as the patient had no history of long-term use of antibiotics, antimalarial drugs, or skin-whitening agents.
Although alkaptonuria does not require any specific treatment, nitisinone is considered a promising remedy.[17] Nitisinone is an inhibitor of 4-hydroxyphenylpyruvate dioxygenase, which converts HGA to pyruvic acid. Nitisinone has been approved for the treatment of alkaptonuria in Europe, as it slows the progression of symptoms. Generally, only symptomatic treatment is administered, and an intake of a low-protein diet and vitamin C supplementation may prevent complications. A low-protein diet aims to reduce the production of HGA by limiting the consumption of tyrosine and phenylalanine. Meanwhile, vitamin C (1 g/day) is believed to reduce the oxidation of benzoquinone acetic acid and decrease the formation of free radicals. However, long-term outcomes are still insufficient.[5]
The treatment for ochronotic arthritis is similar to that for general arthritis. Total knee replacement surgery is possible with both cemented and cementless prostheses. Moreover, successful treatment outcomes have been reported with simultaneous bilateral replacement surgery.[9] The long-term prognosis is similar to that of patients with degenerative joint diseases.[13]
Alkaptonuria is a rare condition worldwide, often diagnosed late because of its slow progression, as symptoms gradually worsen with age. Therefore, it is necessary to diagnose alkaptonuria prior to surgery through a meticulous examination, and caution must be exercised to prevent the weakened tendon from rupturing as a result of excessive traction during surgery. However, if the condition is discovered during the surgery, the procedure can be continued in the same manner as in the general population without changing the surgical technique.
Notes
Ethics approval and consent to participate
This study was conducted in accordance with the principles of the Declaration of Helsinki.
Conflict of interest
No potential conflict of interest relevant to this article was reported.