A Rare Case of Hyperfunctioning Lipoadenoma Presenting as a Cystic Pararthyroid Lesion
Article information
Abstract
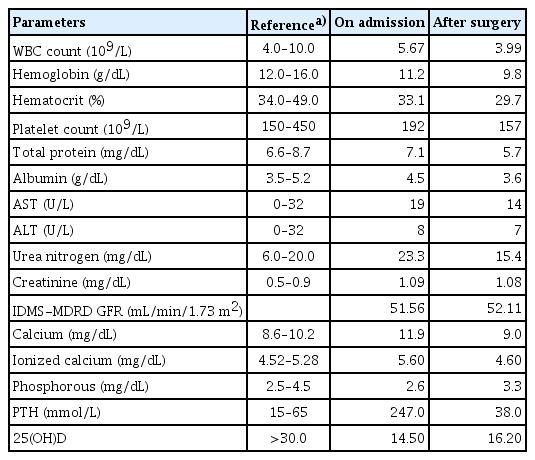
A 58-year-old woman visited the hospital complaining of fatigue and indigestion lasting for more than 3 months. She had no medical history other than taking a calcium plus vitamin D supplement for osteopenia. The initial blood test showed a high calcium level of 14.0 mg/dL. Additional tests were performed to differentially diagnose hypercalcemia. The blood test results were as follows: serum parathyroid hormone (PTH)=247.0 pg/mL, PTH-related peptide <1.0 pg/mL, phosphorous=2.6 mg/dL, 25-hydroxy-vitamin D=14.5 pg/mL, creatinine=1.09 mg/dL, and 24 hr urine calcium=215 mg/dL. A 4.5 cm sized cystic lesion on the intra-thyroidal space was confirmed on neck sonography and 4-dimensional parathyroid computed tomography, but technetium-99m methoxyisobutylisonitrile parathyroid scintigraphy showed equivocal results. After removal of the cystic lesion, serum calcium and PTH were normalized, and parathyroid lipoadenoma was confirmed in the postoperative pathology. Clinical features of parathyroid lipoadenoma are known to be similar to common parathyroid adenoma, but imaging studies often report negative findings. Therefore, it is necessary to better understand this rare disease for the differential diagnosis. For the final diagnosis and treatment of this disease, parathyroidectomy with intraoperative PTH measurement may be required.
INTRODUCTION
Hypercalcemia is easily overlooked because it usually causes non-specific symptoms such as fatigue and constipation.[1] However, it is important to identify hypercalcemia with non-specific symptoms and to diagnose the causative disease in preventing possible chronic complications such as decreased renal function and osteoporosis. Hypercalcemia can have a variety of causes, but more than half of them are due to primary hyperparathyroidism. Primary hyperparathyroidism is a pathological condition characterized by hyperfunctioning parathyroid glands and is suspected when an increased parathyroid hormone (PTH) level accompanies hypercalcemia. Primary hyperparathyroidism is commonly caused by a single adenoma of the parathyroid gland, accounting for more than 80% of cases.[2] A cystic parathyroid lesion is one of the rare causes of primary hyperparathyroidism and may be misdiagnosed as a thyroid cyst, brachial cleft cyst, or parathyroid carcinoma.[3] In the case of cystic parathyroid lesions, up to 85% of cases have been reported as functioning cysts, so careful observation is required.[4]
Here, we report a case of hyperfunctioning parathyroid lipoadenoma diagnosed pathologically after surgery for a cystic parathyroid lesion, which was revealed as the cause of hypercalcemia.
CASE REPORT
A 58-year-old woman visited the hospital complaining of fatigue and indigestion that had lasted for more than 3 months. She had no medical history other than taking a calcium plus vitamin D supplement for osteopenia. The initial blood test showed a high calcium level of 14.0 mg/dL. Additional tests were performed to differentially diagnose hypercalcemia. The blood test results were as follows: serum PTH=247.0 pg/mL, PTH-related peptide <1.0 pg/mL, phosphorous=2.6 mg/dL, 25-hydroxy-vitamin D=14.5 pg/mL, creatinine=1.09 mg/dL, and 24 hr urine calcium=215 mg/dL. Bone mineral density (BMD) was represented by a lumbar T-score=−1.6, femur neck T-score=−1.6, and total hip T-score=−0.4 (Table 1). She reported no specific family history.
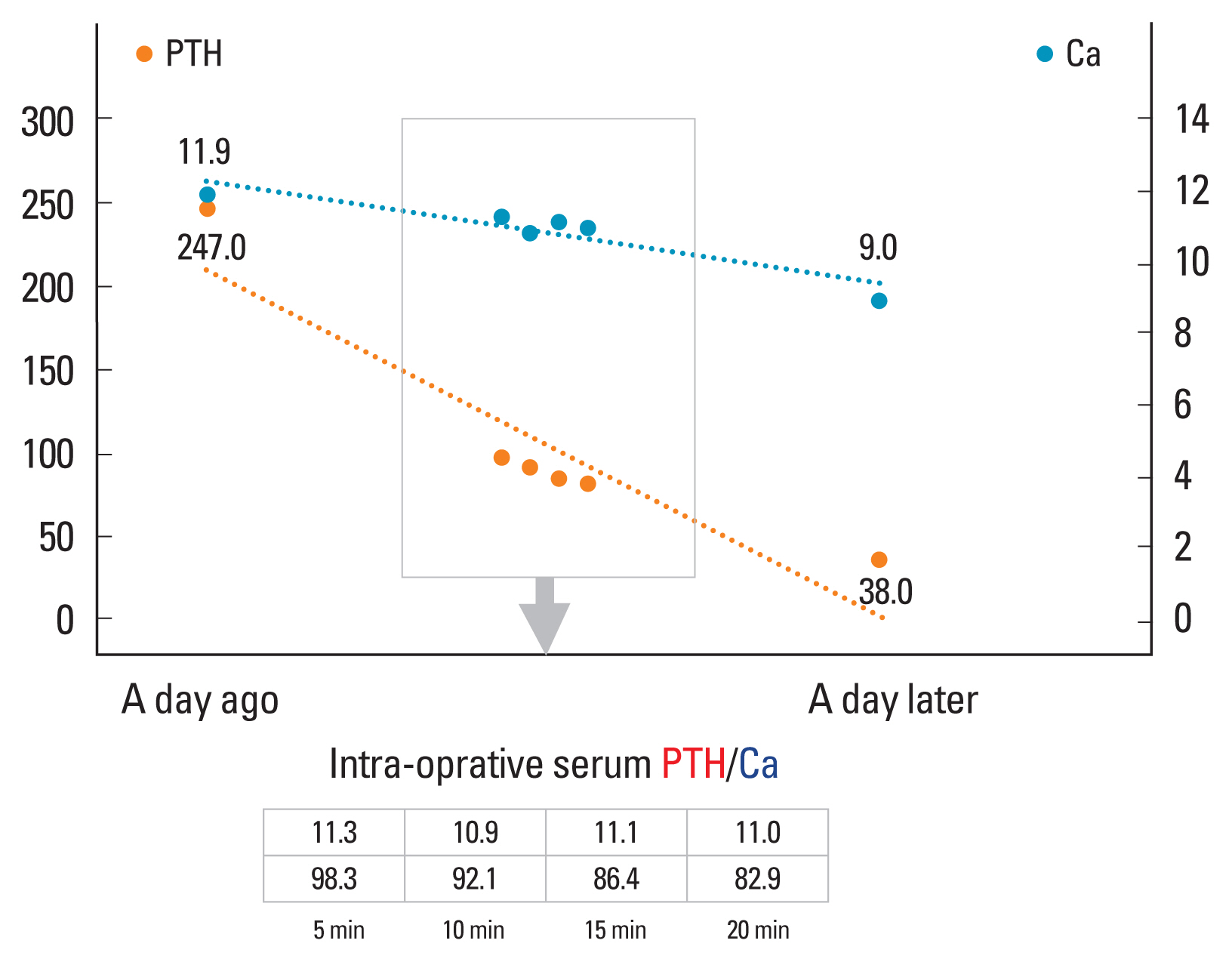
A 4.5 cm sized predominantly cystic nodule located at the right intra-thyroidal space was confirmed on neck sonography and 4-dimentional parathyroid computed tomography (CT), but technetium-99m methoxyisobutylisonitrile (99mTc-MIBI) scintigraphy showed no hyperfunctioning lesion in neck (Figs. 1–3). Despite the discrepancy in imaging findings, we planned surgery for the cystic parathyroid lesion while performing intraoperative PTH measurement. During the surgery, intra-cystic fluid was aspirated for diagnosis, and the PTH concentration of the cystic fluid was confirmed to be as high as 1,294.5 pg/mL. To ensure complete removal of the target lesion, intra-operative serum PTH monitoring was performed 5, 10, 15, and 20 min after resection of the cystic parathyroid, and the levels were 98.3, 92.1, 86.4, and 82.9 pg/mL, respectively, which were less than half of the initial serum PTH level −247.0 pg/mL (Fig. 4).
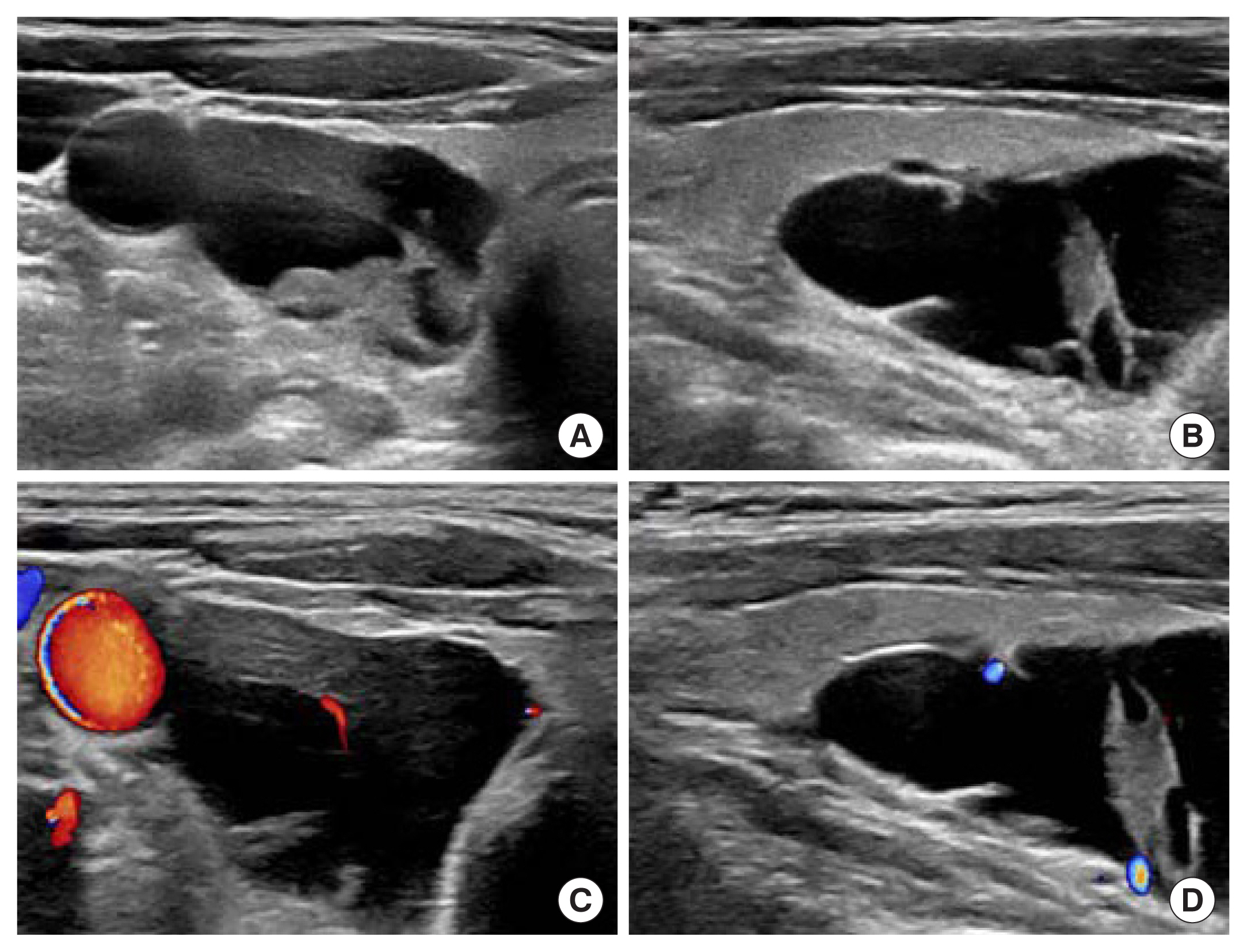
Neck ultrasonography. (A) Transverse view, gray scale, (B) longitudinal view, gray scale, (C) transverse view, color Doppler setting, (D) longitudinal view, color Doppler setting, showing a 2.2×2.5×4.5 cm sized predominantly cystic nodule located at right intra-thyroidal space.
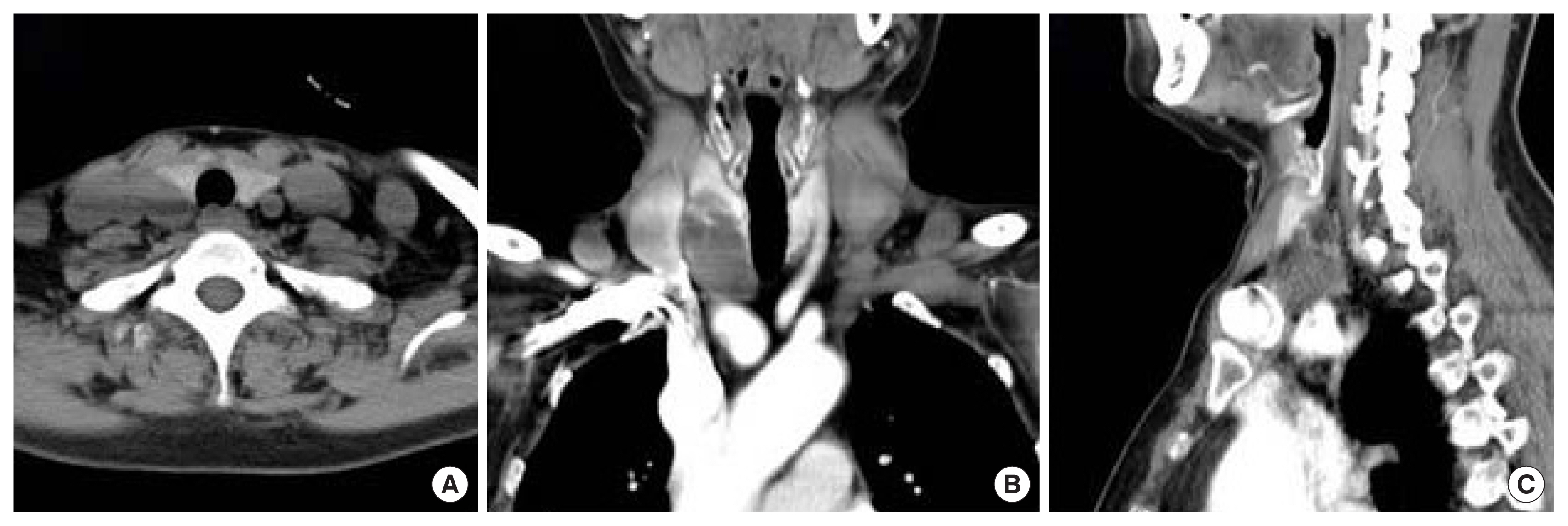
Parathyroid 4-dimensional computed tomography. (A) Pre-contrast transverse view, post-contrast (B) longitudinal view, and (C) sagittal view presenting a lobulated non-enhanced lesion of 2.8 × 2.8 × 4.6 cm in size in the right intra-thyroidal space.
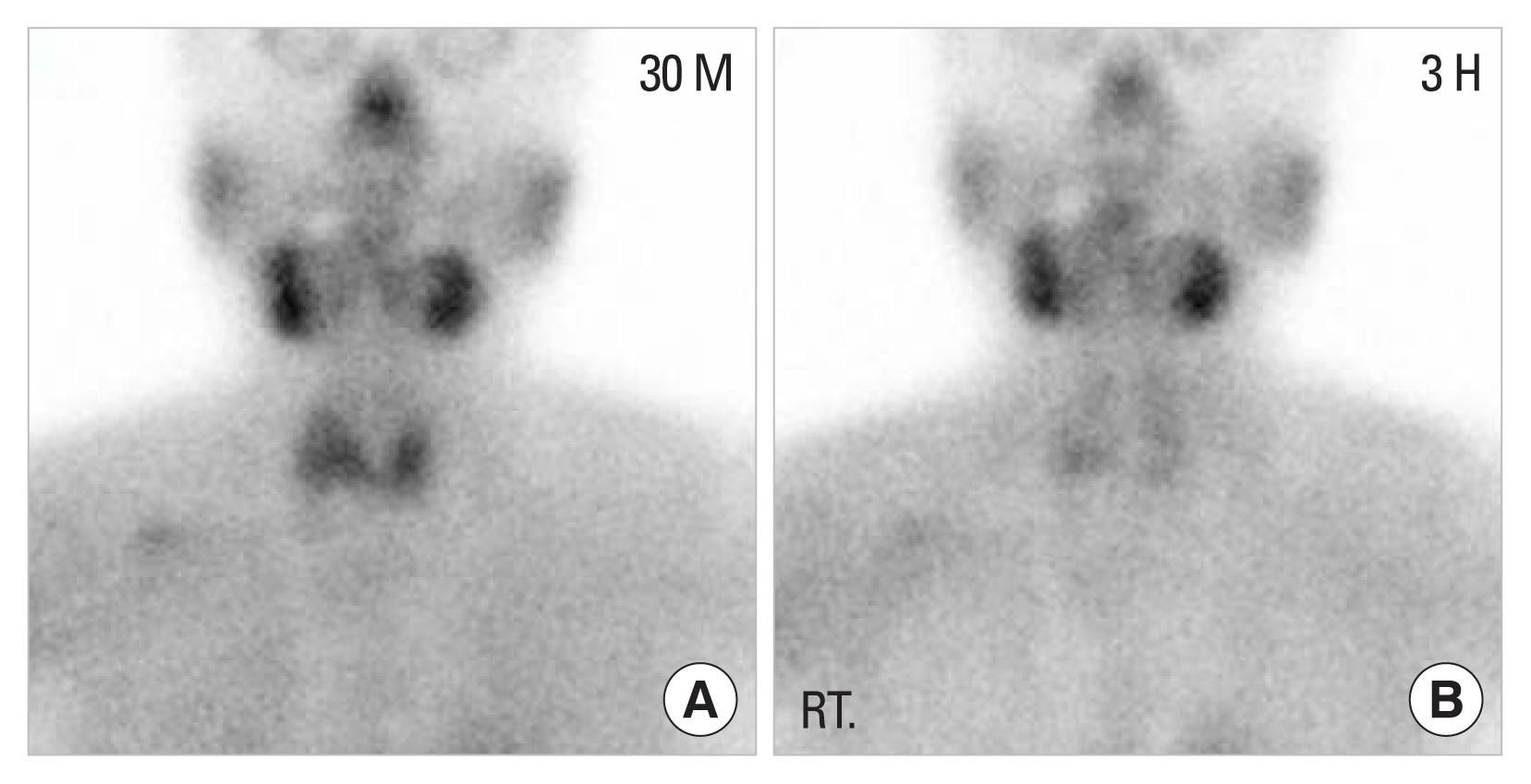
Localization study technetium-99m methoxyisobutylisonitrile parathyroid scintigraphy scan. (A) Early phase, (B) delayed phase, showing equivocal results.
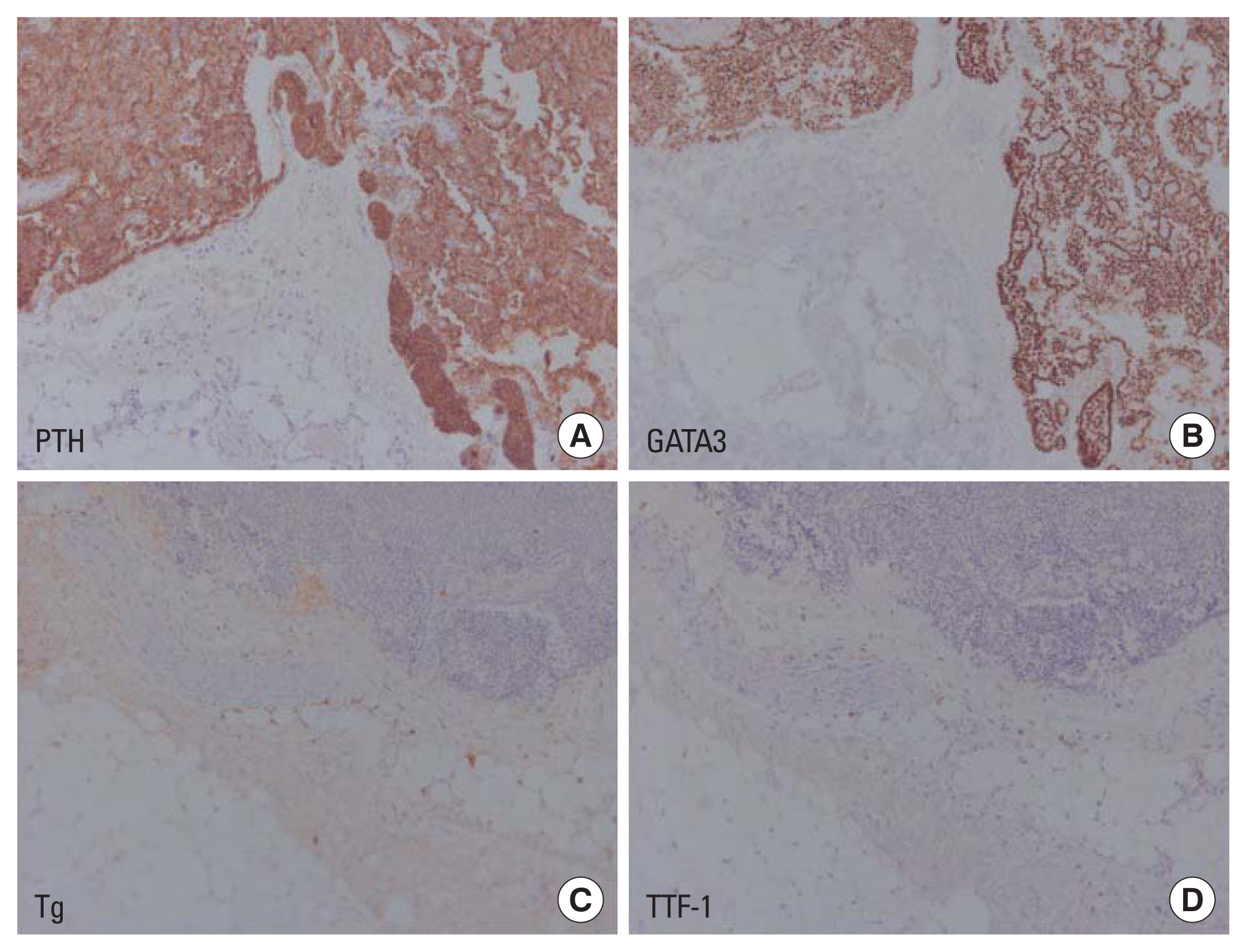
After removal of the lesion, serum calcium and PTH were normalized, and parathyroid lipoadenoma was confirmed with dispersed clusters of follicular structures with abundant mature adipose tissue in the postoperative pathology (Fig. 5). Considering that the cyst was located in the intra-thyroidal space, additional staining was performed to differentiate it from thyroid follicular adenolipoma. Positive findings in PTH and GATA-3 stain and negative findings in thyroglobulin and thyroid transcription factor-1 stain confirmed that the lesion was of parathyroid origin (Fig. 6). Serum PTH and calcium levels measured 3 months after surgery were also confirmed to be normal, and the patient’s hypercalcemia was cured with parathyroidectomy (Table 1).
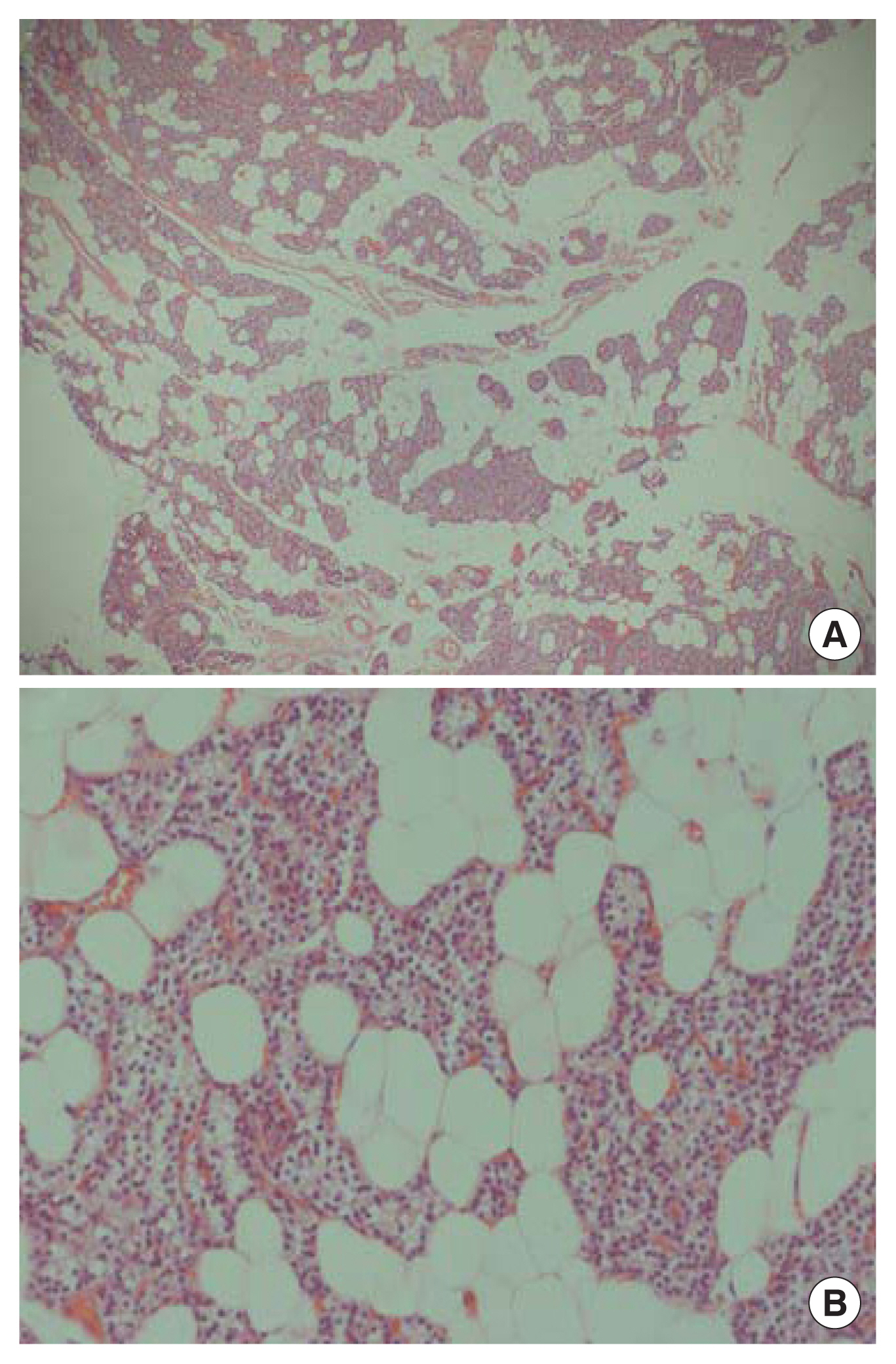
(A) Hematoxylin and eosin (H&E) ×40 magnification. (B) H&E ×200 magnification. The parathyroid mass showed (A) dispersed clusters of follicular structures with abundant mature adipose tissue. (B) Higher magnification of glandular structures revealed minimal atypia and conspicuous nuclear size variation, without mitosis.
DISCUSSION
Most patients with primary hyperparathyroidism are asymptomatic, and blood or urine calcium levels remain normal for a considerable period of time.[5] Therefore, symptoms such as severe hypercalcemia (increased by more than 1 mg/dL above the normal range), decreased renal function, and recurrent kidney stones are indications for surgical treatment.[6] Bone health may also be the one of important considerations in primary hyperparathyroidism. The increased state of PTH accelerates bone turnover, leading to bone loss, and consequently the prevalence of primary hyperparathyroidism is high in patients with low BMD.[7] Therefore, it is necessary to check the serum PTH level because of the possibility of secondary osteoporosis in patients with osteoporosis.
There can be a variety of causes of elevated serum PTH.[8] Hyperparathyroidism is classified as primary hyperparathyroidism when the cause of elevated serum PTH is due to an abnormality of the parathyroid gland itself, and secondary hyperparathyroidism when the increase in serum PTH is due to another medical condition, such as renal disease or vitamin D deficiency. Tertiary hyperparathyroidism, which occurs due to sustained stimulation of the parathyroid glands due to long-term deterioration of renal function, is often mistaken for primary hyperparathyroidism, so careful observation is required in patients with reduced renal function. In pseudohypoparathyroidism, a disease caused by a genetic abnormality of the PTH receptor, elevated PTH and hypocalcemia are observed simultaneously.[9] In our case, hypercalcemia was accompanied by increased PTH, while renal function and vitamin D levels were within normal ranges, suggesting primary hyperparathyroidism.
To find parathyroid lesions that cause primary hyperparathyroidism, neck sonography can be performed to confirm anatomical abnormalities of the neck. Parathyroid lesions are mainly observed as hypoechoic lesions adjacent to the capsule of the echogenic thyroid gland, but they are not seen in the general population. Therefore, pathologic diseases should be suspected if a parathyroid lesion is found on sonography. Additionally, since parathyroid cysts are very rare, they can be misdiagnosed. Clinical characteristics of parathyroid cysts to keep in mind are that they occur mainly in middle-aged women, they are found in the mid or lower pole area of the thyroid gland, and they have an echogenic border between the thyroid gland, as in this case report.[10] Differential diagnoses to be considered, thyroglossal duct cyst and branchial cleft cyst, are located in the midline of the neck or deep in the lateral neck, near the pharyngeal wall, respectively. Fatty tissue is common to be observed hyperechoic than the surrounding muscles, and it has been reported that parathyroid lipoadenoma can also be showed as a hyperechoic lesion in a previous case series.[11] However, in this case, the lesion observed on ultrasound is a hypoechoic lesion in the intra-thyroidal space, making it difficult to clearly differentiate it from a thyroid cyst.
The 99mTc-MIBI scintigraphy, a functional imaging test, can be performed to localize pathological parathyroid lesions. The diagnostic accuracy of primary hyperparathyroidism using a MIBI scan is about 85% when the serum PTH level is 150 ng/dL or higher; the accuracy may be lower in milder forms of the disease.[12] When considering the sonography results, the accuracy can be increased,[13] and it is suggested that single photon emission CT/CT fusion imaging or 4-dimensional CT can be used for more accurate preoperative diagnosis.[14] In the preoperative condition, fine needle aspiration (FNA) may increase the risk of hemorrhage, parathyromatosis, or tumor seeding, particularly in the case of parathyroid carcinoma. Parathyroid cancer may have a relatively large size, inhomogeneous contents, and lobulated contours. However, parathyroid adenomas may also present cystic changes, calcifications, and lobulations as it grows in size.[15] Therefore, the guidelines of the American Association of Endocrine Surgeons do not recommend FNA for parathyroid lesions.[16]
The treatment of primary hyperparathyroidism is surgical removal of the hyperfunctioning parathyroid lesion. If the causative lesion is localized before surgery, the surgical plan is likely to be clear and safe. However, even if the pathologic parathyroid gland is not clearly localized before surgery, it is necessary to perform exploratory surgery while checking the intraoperative serum PTH levels. In addition, intraoperative PTH is a useful test even in cases that appear as a single lesion on image findings because there are cases in which multiple lesions may actually be present. Treatment failure due to residual parathyroid glands can be prevented by confirming intraoperative PTH reduction after removal of the parathyroid.[17] In our case, the serum PTH concentration and calcium concentration were monitored before, during, and after surgery, and treatment went well without any other symptoms or complications.
In the case of functional parathyroid cysts, it is hypothesized that they are caused by hemorrhage and cystic degeneration of parathyroid adenoma.[18] However, most case series have been from very small studies, and there are few cases in which gross and microscopic pathologies have been matched with imaging findings. Parathyroid lipoadenoma was first reported as hamartoma in 1958,[19] and has been reported as lipohyperplasia, myxoid stroma, or lipoadenoma.[20] Its prevalence is thought to be very low at 0.2%, and fewer than 80 cases of parathyroid adenoma have been reported worldwide.[21] Clinical features of parathyroid lipoadenoma are known to be similar to common parathyroid adenoma, but imaging studies often report negative findings because of the high-fat content.[22] Therefore, it is necessary to understand this rare disease for the differential diagnosis. For the final diagnosis and treatment of this disease, parathyroidectomy with intraoperative PTH measurement is required.[23]
In conclusion, cystic parathyroid lesions could be one of the causes of primary hyperparathyroidism and may be misdiagnosed as thyroid cysts, brachial cleft cysts, or parathyroid carcinoma. Cases of pathologically confirmed lipoadenoma are also very rare. We reported a cystic parathyroid lesion revealed to be a hyperfunctioning lipoadenoma to promote understanding of the various types of primary hyperparathyroidism.
Notes
Authors’ contributions
Conceptualization, KHS; investigation, JK and OK; writing—original draft preparation, JK and OK; writing—review and editing, JK, OK, TJK, SLJ, EJH, and KHS. All authors have read and agreed to the published version of the manuscript.
Ethics approval and consent to participate
This case report was confirmed by the Institutional Review Board of Yeouido St. Mary’s Hospital (SC23SISI0052).
Conflict of interest
No potential conflict of interest relevant to this article was reported.