Position Statement: Exercise Guidelines for Osteoporosis Management and Fall Prevention in Osteoporosis Patients
Article information
Abstract
Background
The effectiveness of exercise for improving osteoporosis and fall prevention in patients diagnosed with osteoporosis or osteopenia has not been fully summarized. The Korean Society for Bone and Mineral Research and the Korean Society of Exercise Physiology has developed exercise guidelines for patients with osteoporosis or osteopenia and provide evidence-based recommendations.
Methods
A systematic review identified randomized controlled trials (RCT) assessing the effect of resistance, impact, balance, aerobic training, and physical activity in osteoporosis and osteopenia on bone quality, physical performance, quality of life, and fall prevention. PubMed, Embase, KoreaMed, and RISS were searched from January 2000 to August 2022. Ten key questions were established to review the evidence and formulate recommendations.
Results
The 50 RCTs reported that even with osteoporosis and osteopenia, resistance and impact training consistently maximized bone strength, improved body strength and balance, and eventually reduced fall incidences. Resistance exercise combining 3 to 10 types of free weight and mechanical exercise of major muscle groups performed with an intensity of 50% to 85% 1-repetition maximum, 5 to 12 repetitions/set, 2 to 3 days/week, for 3 to 12 months is recommended. Impact exercises such as jumping chin-ups with drop landings and jump rope performed 50 jumps/session for at least 6 months with 3 or more days/week are recommended.
Conclusions
A multi-component exercise mainly comprised of resistance and impact exercise seems to be an effective strategy to attenuate the risk factors of osteoporosis and osteopenia. The integration of exercise guidelines and individualized exercise plans has significant potential to reduce the morbidity and mortality of osteoporosis.
INTRODUCTION
Osteoporosis is a common systemic disease characterized by reduced bone mass and deterioration of bone microarchitecture, leading to increased bone fragility and the risk of fractures.[1] Fractures in individuals with low bone mass are commonly caused by falls,[2] which can lead to pain, decreased mobility, and a reduction in both physical and social functional capacity.[3,4] In particular, following hip and vertebral fractures, common in osteoporosis patients, increase the risk of subsequent fractures and mortality.[5–7] Although pharmacological options for improving osteoporosis and fracture prevention are available, non-pharmacological approaches, such as exercise, are required to attenuate bone loss and the risk of falls for a better quality of life and to reduce the healthcare burden.
Several studies and guidelines suggested that regular exercise can effectively preserve or augment bone mineral density (BMD), which is known to reduce the risk of osteoporosis and fall-related fractures.[8,9] In addition, osteogenesis occurs via mechanical stimuli [10] such as the impact on the bone surface or contraction of surrounding muscles.[11] Mechanical stimuli by resistance training, weight-bearing or impact exercises, and balance training are known for their favorable exercise modes to enhance bone health and augment muscle mass and strength, which improves posture balance and stability and reduces the risk of falls over time for older individuals.[10–14] However, a lack of evidence exists regarding the benefits versus harmfulness or no effects of prescribing those exercises to post-menopausal women,[14] individuals with osteoporosis,[15] or people at moderate to high risk of fractures. Despite clinical practice guidelines for osteoporosis patients recommending exercise as a positive tool to prevent fractures, the efficacy of exercise may vary by type of exercise, study population, or outcomes of interest. In turn, although exercise has been proposed as a potential strategy to manage osteoporosis, there is no consensus on the most promising types of exercise for improving bone mass.
The American College of Sports Medicine (ACSM) has published guidelines for physical activity (PA) and bone health and recommended that healthy adults must perform a jumping exercise, weight-bearing aerobic exercises for at least 3 to 5 days per week, and resistance exercise at moderate to high intensity for 30 to 60 min per day for 2 to 3 days per week to maintain bone health.[16] Although the influence of exercise on bone metabolisms is relatively well-understood, and previous guidelines recommend engagement in exercise for the management of osteoporosis, the effectiveness of exercise for improving osteoporosis and fall prevention in patients diagnosed with osteoporosis or osteopenia has not been summarized. Specifically, uncertainty still exists on whether increasing exercise intensity and volume in osteoporosis patients would improve bone strength and fall prevention, and prominently, which types of exercise intervention or degrees of exercise intensity are most beneficial. In the past, exercise recommendations for individuals with a high risk of osteoporosis were generated by studies with age-matched healthy participants.[17] To date, an increasing number of randomized controlled trials (RCTs) examined the impact of various types of exercise on the subject focusing on osteoporosis patients in the past decade. Integrating the data into evidence-based recommendations is needed both to prevent fracture incidences and rehospitalization due to worsening osteoporosis and to offer orthopedic providers a guideline to help patients navigate the correct information regarding exercise for patients with osteoporosis. Therefore, the Korean Society for Bone and Mineral Research (KSBMR) and the Korean Society of Exercise Physiology (KSEP) exercise guideline seeks to provide evidence-based recommendations to improve bone health and fall prevention in patients with osteoporosis or osteopenia.
METHODS
The guidelines were developed to provide a more systematic approach to exercise for the management of osteoporosis and fall prevention in osteoporosis patients. The KSBMR and KSEP have collaboratively formed a development committee (exercise committee) aimed at establishing exercise guidelines for the management of osteoporosis and fall prevention. The committee is an expert in multidisciplinary fields and multi-institutional, which consists of exercise physiologists, on-site exercise specialists, physicians, and orthopedic surgeons. Members of the committee were required to contribute to development of guidelines, provide critical reviews, and finalize the exercise guidelines recommendations in consideration of the evidence-based on the pre-selected key questions (KQs) to define the effects of exercise for the final selection of exercise methods. The literature was chosen using a systematic review as described below.
1. Framework for guideline development
The guideline development process consisted of 3 phases: planning, development, and announcing (finalization).[18,19] Briefly, a total of 12 individual steps were applied. The first phase consisted of (1) theme selection; (2) organizing the development committee; (3) review of previously published guidelines; (4) development plan establishment; and (5) the KQs selections. The second phase of the development phase consisted of (6) evidence retrieval; (7) evaluation; and (8) synthesis; (9) formulating recommendations and establishing the recommendations grade; and (10) committee’s consensus attaining. Lastly, phase 3, consisted of (11) external review; and (12) publication.
2. Selection of KQs
The committee established the KQs by reviewing the position statement published in USA,[16] Canada,[9] and Australia.[20] Furthermore, the Physical Activity Guidelines for Americans (2nd edition) published by US Centers for Disease Control and Prevention,[21] and numerous position statements in the United Kingdom,[22] Europe,[23] and USA [24] were referenced. Based on reviewing other position statements, the committee initially nominated the 13 most pertinent KQs, which account for the domestic situation and clinical significance, and finally listed 10 KQs (Table 1). The exercise method in the KQ included general exercise prescription factors, including frequency, intensity, time, and type (FITT).
3. Literature search
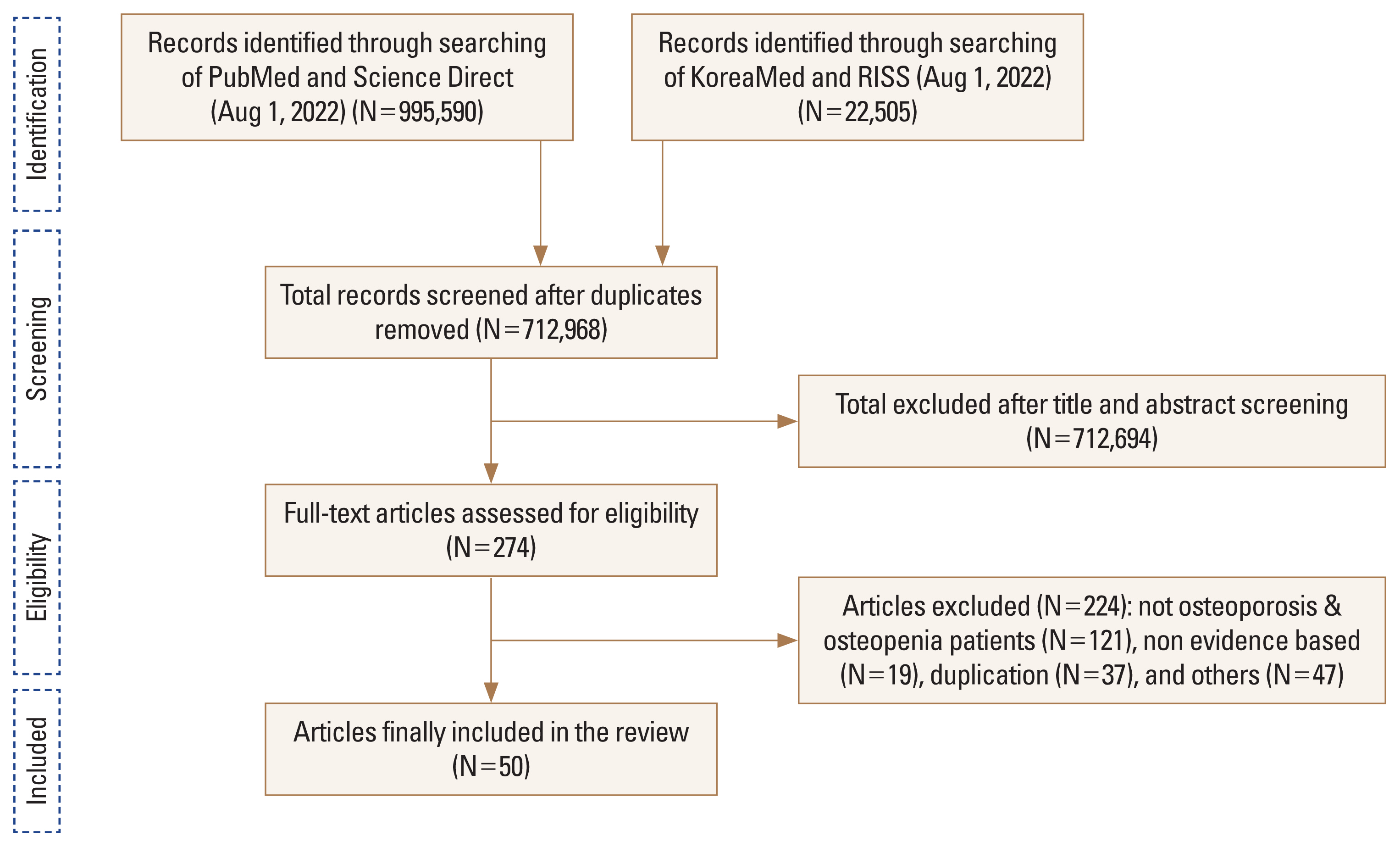
To answer the KQs, an extensive literature search was conducted by using a systematic review of evidence identified through online databases such as the PubMed of the US National Library of Medicine, Embase of Elsevier, KoreaMed of the Korean Association of Medical Journal Editors, and RISS of the Korea Education and Research Information Service. Only data from RCTs published from January 1st, 2000, through August 1st, 2022, were considered. The keywords for the literature search were described in the Supplementary Appendix 1. In the review stage, the committee used the Participants, Intervention, Comparison, Outcomes, Time, Setting, and Study Design (PICOTS-SD) framework to determine the scope of inclusion in the literature for the use of systematic review methods (Supplementary Table 1).[25] Among a million outcomes, duplicate studies were excluded using the program EndNote (Clarivate Analytics, Philadelphia, PA, USA) and firstly yielded 712,968 relevant articles without duplicates. The initially selected articles were further reduced down to 274 of the most relevant papers, of which 50 were finally selected, excluding non-osteoporotic and osteopenic patients (121 cases), non-evidence based (19 cases), duplication (37 cases), and others (47 cases) (Fig. 1). The finally chosen papers were experimental papers with randomized-controlled trials study design with clear PICOTS-SD, which allowed to provide the evidence-based recommendation about KQs selected by the committee.
4. Quality appraisal (Risk of bias)
The RCTs finally included in the review were further assessed for their quality using the Cochrane Collaboration’s tool by measuring the risk of bias from the 2 independent reviewers.[25] Any conflicts were discussed between review authors and then confirmed and finalized. The quality items were the following: (1) potential randomization process bias; (2) deviations from intended interventions bias; (3) missing outcome data bias; (4) outcome measurement bias; and (5) selecting the reported result bias.[25] The review authors rated each entry as “low risk,” “high risk,” or “unclear risk.” Supplementary Figures 1 and 2 show the results of the risk of bias assessment for each study according to the domain. Overall, the general risk of bias was low to moderate in the included studies.
5. Procedures for conclusive selection and formulation of guidelines
Following the review of the evidence from the conclusively selected works of literature, the committee reviewed the capability to apply the guideline for each KQ by summarizing the contents of the selected evidence as a response to the KQ. The comments from the committee members were subsequently collected and completed the recommendation. Based on the applicability review, ratings for the type of evidence, the strength of evidence, and the grade of recommendations were provided. The rate of evidence was categorized into 4 levels (Supplementary Table 2), and the recommendation grade was established according to the committee’s consensus with the principle of 80% or more (Supplementary Table 3).
RESULTS
1. KQ1. Is resistance training an effective strategy for improving osteoporosis and fall prevention?
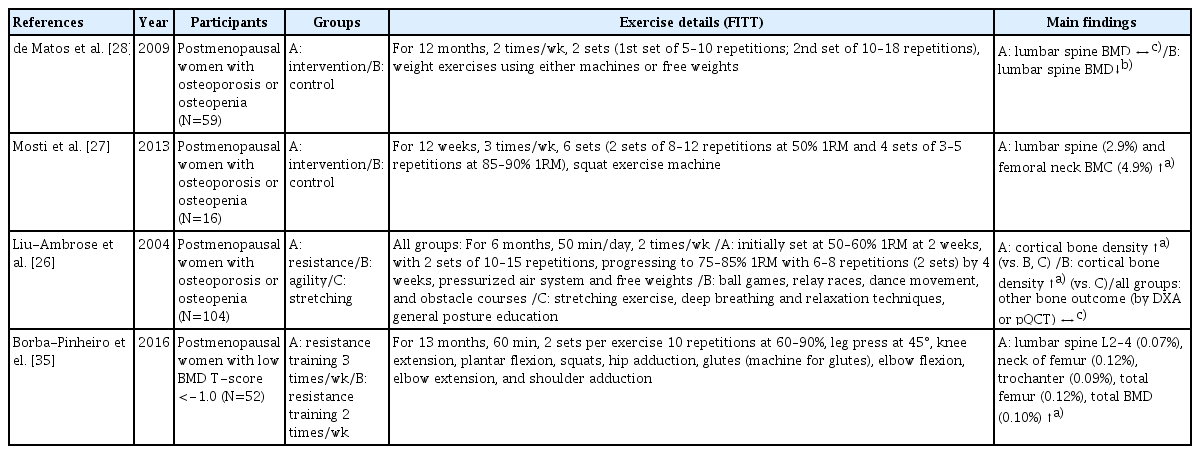
Resistance training has been recognized as an effective method to improve bone health by avoiding decreases in BMD over time for individuals with osteoporosis or osteopenia (Table 2). Liu-Ambrose et al. [26] reported significantly increased cortical bone density at the radial shaft among 75- to 85-year-old women with diagnosed osteoporosis or osteopenia who performed 6 months of resistance training. Mosti et al. [27] also showed that 3 months of squat exercise using a machine in postmenopausal women with osteoporosis or osteopenia significantly increased bone mineral content (BMC) at the lumbar spine and femoral neck by approximately 2.9% to 4.9%. Furthermore, de Matos et al. [28] evaluated the effects of resistance training using machines and free weights on BMD of postmenopausal women with osteoporosis or osteopenia, thereby non-exercising women exhibited a significant percentage decrease in BMD at the lumbar spine, while lumbar spine BMD maintained in the group of 12 months of weight training intervention. Similar findings were observed in older men with osteoporosis or osteopenia utilizing high-intensity resistance training.[29,30] Based on the results of the studies outlined in Table 2, resistance training might be protective against further reductions in bone mass and plays a beneficial role in reducing osteoporosis-related fracture risk among those already diagnosed with osteoporosis or osteopenia.
2. KQ2. What type of resistance training is effective for improving osteoporosis and fall prevention?
To attain the maximal benefits of resistance training in individuals with osteoporosis or osteopenia, careful manipulation of training variables, such as intensity, volume, and frequency, is required.[31] Traditional resistance training recommendations suggest an intensity of below 60% 1-repetition maximum (1RM) in older healthy adults to improve muscular function.[32] However, as shown in Table 2 in the articles with osteoporosis patients, participants initially performed 2 sets of 10 to 12 repetitions at 50% 1RM as a warm-up and gradually progressed to 85% of 1RM at 5 to 8 repetitions based on the patient’s condition.[26,27, 29,31,33] Concerning resistance training intensity, the majority of studies indicate that exercises generating high-intensity loading forces (70%–90% 1RM) are more effective for increasing BMD among postmenopausal women.[12] The number of training sessions in a given period can also determine the training benefits and recovery from acute exercise, especially in the older population.[34] For instance, Borba-Pinheiro et al. [35] reported that resistance exercise 3 times a week among postmenopausal women with low BMD showed better results for all BMD variables than twice a week when exercise type, intensity, and time were identical. Therefore, 3 times a week is recommended as a time-efficient resistance training strategy to prevent age-related bone loss and falls. However, resistance training frequency should be considered in conjunction with training volume, as training volume can determine the recovery required after vigorous training.[34]
3. KQ3. Are impact exercises effective for improving osteoporosis and fall prevention?
Several studies suggested that mechanical loading induced by exercise training has a positive effect on the skeleton by generating an optimal stimulus.[36] Although few studies confirmed the effect of impact exercise alone in osteoporosis patients, several studies have reported the effect of improving BMD, fall risk, and functional fitness in osteoporosis through impact exercise combined with resistance training (Table 3). For example, a study by Basat et al. [37] showed that 6-month high-impact exercise training significantly increased BMD due to increased bone formation marker and decreased bone resorption marker compared to the strengthening exercise and control groups in postmenopausal women with osteopenia. Impact exercise training combined with resistance training for 8-month increased BMD, functional performance, and muscle strength, but BMD didn’t change when the duration was less than 8-month.[31,33,38–41] Additionally, 12-month aerobic plus impact exercise intervention showed higher femoral neck strength and BMC than a group with usual activities.[42,43] Therefore, the aggregated data suggest that impact exercise training can be an effective method to prevent bone loss in older women.
4. KQ4. How can impact exercises be effectively applied to improve osteoporosis and fall prevention?
Although impact exercise training has been understood as an effective way to improve BMD and fracture risk in women with osteoporosis, an accurate FITT for impact exercise has not been presented. As shown in Table 3, the intervention with impact exercise, especially jumping exercises such as jump rope, jump drop, and jumping chin-ups with drop landing, were applied to women with osteopenia or osteoporosis with a duration varying from 6- to 12- months. Most studies suggest that performing impact exercise at least 2 to 3 times a week improves BMD. The intervention consisting of only impact exercise training for 6 months was sufficient to change BMD, while longer than eight months was required when impact exercise training combined with resistance or aerobic exercise to generate similar outcomes. Given the totality of the previous outcomes, impact exercise training with suitable exercise duration is a powerful exercise regimen to improve bone health in patients with osteoporosis.[33,37,38,42–44]
5. KQ5. Are balance exercises an effective strategy for improving osteoporosis and fall prevention?
In individuals with osteoporosis or osteopenia, strategies to prevent falls and fall-related fractures are even more essential than in healthy individuals. As stated above section, older women with osteoporosis show decreased balance and muscle strength, which causes a higher risk of falling, and further increased fracture risks compared to age-matched individuals.[7] Several reports have suggested the effects of balance exercises on osteoporosis and fall prevention (Table 4). For example, the balance training for 12 months improved functional balance and decreased the number of fall incidences in patients with osteoporosis.[45,46] A relatively short period (5.5 or 12 weeks) of combined exercise, including weight-bearing and walking exercise, has shown decreased fall rates in patients with osteoporosis after the intervention.[47] In older women with osteopenia, resistance exercise and agility training for 50 min per day, 2 days a week for 24 to 25 weeks, reduced the risk of falling.[48] Overall, RCTs confirmed that the improved postural balance following exercise training could reduce the risk of fractures by enhancing postural stability and diminishing the risk of falls in older women with osteoporosis.[49–51] Therefore, both balance training sole and combined exercise that includes balance training are associated with a significantly reduced frequency of falls in osteoporotic patients.
6. KQ6. What is the effective way of applying balance exercises to improve osteoporosis and fall prevention?
As shown in Table 4, the intervention in osteoporotic patients with balance training for 30 to 60 min per day at least 1 to 2 days per week for 48 weeks resulted in a reduced risk of falls and functional balance.[45,46] Balance training should progressively proceed from easy to demanding exercise as the physiological strength of training increases over time. Combined exercise programs, including weight-bearing exercise, walking exercise on an obstacle course, and correction of gait abnormalities with 15 to 90 min per day, once a week for 5.5 weeks, have shown decreased fall rates and increased BMD.[47] Also, an intervention study of a 10-week (60 min per day for 2 days a week) combined strength and balance training program reported improved static and dynamic balance.[49] The strength training and aerobic exercise program for 24 weeks with 60 min per day, 3 times a week improved postural sway and walking ability.[51] However, the effects of balance training on the risk of falls should be carefully interpreted since the effectiveness of this intervention has been demonstrated when it is combined with other types of exercise, such as strength, weight-bearing, and aerobic exercise.
7. KQ7. Are weight-bearing aerobic exercises effective for improving osteoporosis and fall prevention?
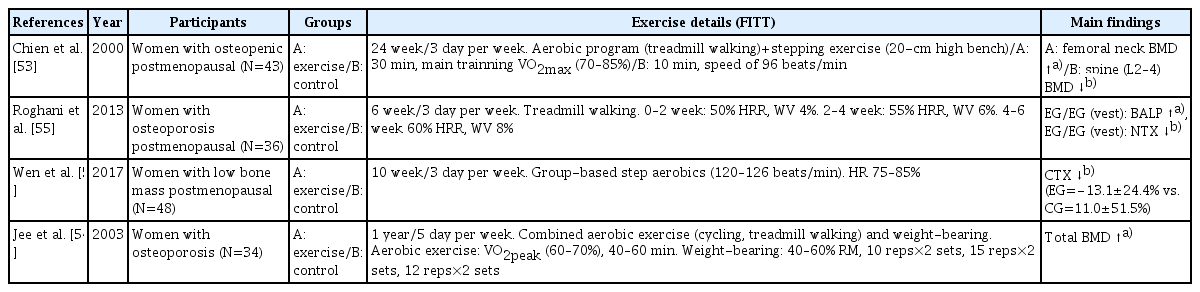
Weight-bearing aerobic exercise refers to a form of aerobic exercise performed mainly using the lower extremities while moving bones and muscles against one’s weight gravity. A representative form of weight-bearing aerobic exercise is walking, jogging, stair climbing, dancing, volleyball, tennis, and Tai Chi.[52] Studies on osteoporosis patients also reported that an exercise program in combination with aerobic and anaerobic exercise was positive for improving BMD and bone metabolic biomarkers (Table 5).[53–56] In particular, a study by Chien et al. [53] showed the improved BMD of the femoral neck in osteoporosis patients who performed both treadmill and stepping exercises. Taken together, a walking exercise could be the most simple and self-manageable way of preventing osteoporosis fractures, at least in terms of bone mass and BMD maintenance. However, since the effect of improving bone density may be limited only by walking exercise, it will be a more effective strategy for bone health if combined with impact exercise or resistance training.
8. KQ8. How can weight-bearing aerobic exercises be effectively applied to improve osteoporosis and fall prevention?
Most studies with weight-bearing aerobic exercise have been conducted with combined exercise, mainly treadmill walking, cycling, and resistance training. In a study by Chien et al. [53], walking exercise at moderate to the vigorous intensity combined with step exercise 3 times a week for 24 weeks caused a significant increase in femoral neck BMD. Two studies also revealed that combined exercise (cycling or treadmill walking + weight training) 5 times a week for a year increased total BMD,[54] pelvic, spine, and trunk BMD.[57] However, similar studies with relatively short duration (6–12 weeks) did not show beneficial effects in BMD, whereas the specific markers of bone formation include bone specific alkaline phosphatase, irisin, and osteocalcin were significantly increased, while the marker of bone resorption such as N-terminal telopeptide of collagen type I and C-terminal telopeptide were decreased.[55,56,58] Furthermore, weight-bearing aerobic exercise with weighted vests, even for 6 weeks, was effective in improving bone metabolic markers.[55] We also recommend outdoor exercise at a moderate intensity of the rate of perceived exertion (RPE) 11 to 14 or 40% to 60% of the maximum heart rate for 30 min a day, at least 3 times a week.
9. KQ9. Are PA effective for improving osteoporosis and fall prevention?
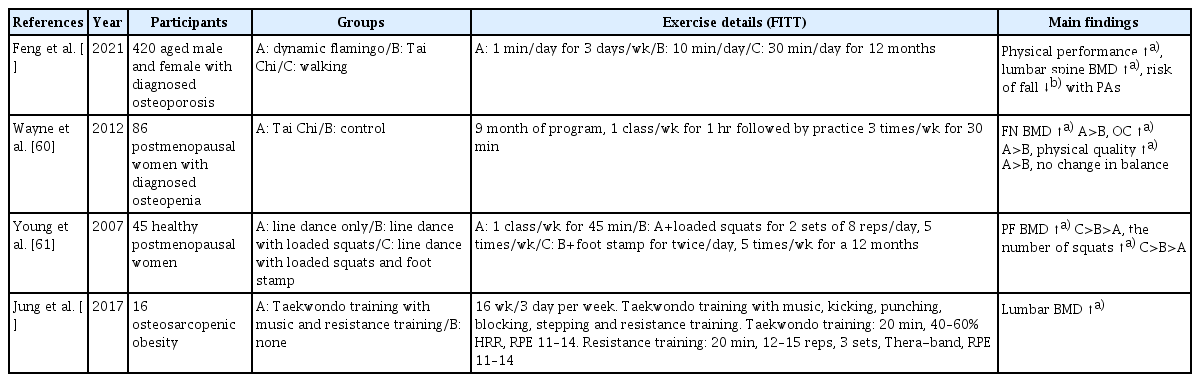
It has long been known that various PA including Tai Chi, dance, and yoga help bone regeneration, development, and positively associated with higher BMD. Some studies have reported the effects of fracture prevention through PA (Table 6). Multiple types of PA with vitamin D supplementation in osteoporosis patients have shown improved physical performance, including standing balance, 4 m walking, and grip strength after 6 month and 12 months.[59] Also, lumbar vertebral BMD was significantly increased, while the risk of fall was reduced at 12 months.[59] A mind-body PA for 9 months showed increased femoral neck BMD and upregulated serum bone formation marker osteocalcin. Moreover, self-reported physical quality was improved, while there was no change in overall balance.[60] Postmenopausal women with regular PA for 1 year showed increased proximal femur BMD and physical performance.[61] Taekwondo training with music and resistance training was effective in improving lumbar BMD.[62] Therefore, it is plausible that regular PA presents indirect evidence that bone density reduction due to aging or osteopenia can be alleviated and fracture can be prevented.
10. KQ10. What is the effective way of applying PA to improve osteoporosis and fall prevention?
An important question that remains to be further identified is which type of PA positively regulate bone metabolism, BMD, and further prevent risk of fall. In age-associated osteoporosis and osteopenia, various types of PA have been explored including Tai Chi, engaging social activity, line dancing with a combination of foot stamping activity, and Taekwondo training with kicking, stepping.[59–62] The importance of these types of PA in the BMD and fall-related fracture in osteoporosis or osteopenia were described where regular PA at least 6 months at least maintain bone mass and BMD, and additional loading and strain confer the larger positive effects on BMD and physical strength. It is shown that any PA can maintain or increase bone density to some extent, but the anabolic activity of the bone appears to be activated when additional loads or even a little more force are applied in addition to the basic activity.[63] Therefore, our aggregate review suggest that regular PA would be the effective method to maintain BMD and prevent fall-related fracture in patients with osteoporosis and osteopenia, but it could be strengthened particularly in concert with an extra resistance and light impact that may reduce musculoskeletal deficits.
DISCUSSION
The primary goal of establishing the exercise guideline is to provide evidence-based recommendations to improve BMD and fall prevention in patients diagnosed or undergoing treatment with osteoporosis or osteopenia. Most importantly, this guideline is the first to demonstrate specifically the methods and effects of various exercises on the osteoporosis care trajectory in Korea. The guideline showed the effects of exercise based on the criteria of evidence level and the grade of recommendation by selecting effective exercise methods. Table 7 summarized the finalized exercise guideline based on FITT for improving osteoporosis and fall prevention in individuals diagnosed with osteoporosis or osteopenia.
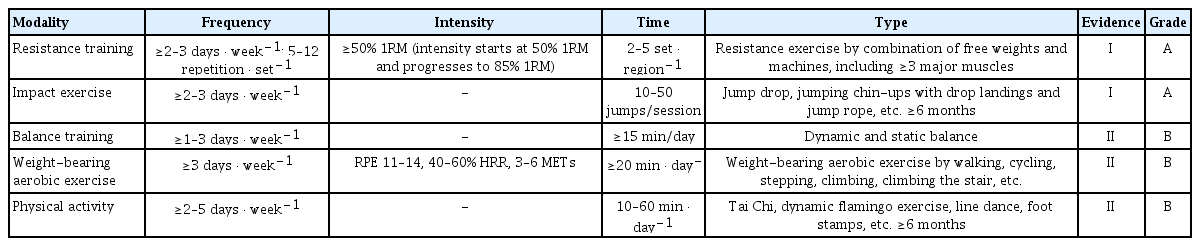
Exercise guidelines based on frequency, intensity, time, and type for improving osteoporosis and fall prevention
This guideline recommends that resistance training and impact exercise appear to be more effective for improving bone mass and preventing the risk of falls, with the highest level of evidence and grade of recommendations. The committee’s review of the evidence suggests that a resistance training program is effective for older adults who are diagnosed with osteoporosis or osteopenia when they performed at least 2 or 3 times a week, with one set of 5 to 12 repetitions by a combination of free- and machine-weight including 3 to 10 exercise in major muscle groups at 50% to 85% 1RM for 3 to 12 months (Evidence level: I /Grade of recommendation: A). However, in clinical practice, a careful evaluation must precede the exercise prescription in patients with osteoporosis and older adults, thus, it is indispensable to identify the individualized type, intensity, and duration of a proposed exercise program. In general, the ACSM guidelines recommend that older adults perform resistance training on at least 2 non-consecutive days each week, with one set of 10 to 15 repetitions for 8 to 10 exercises involving all the major muscle groups at 50% to 60% of the 1RM. In addition, very deconditioned older adults, such as osteoporotic patients, could start resistance training with a “very light” to “light” intensity (40%–50% 1RM) to increase strength, power, and balance.[32] Resistance training is an exercise that improves muscular strength and endurance by contracting a specific muscle or muscle group against external resistance, including free weights, weight machines, and bodyweight exercises such as push-ups or squats. The ACSM now recognizes that resistance training also has a positive effect on bone health across the age spectrum.[32] Moreover, the majority of evidence indicates that resistance training has positive impacts on bone mass in those already diagnosed with osteoporosis or osteopenia by markedly inhibiting aging-associated bone loss, or rather achieving dramatic increases in bone mass.[26–31,33] Likewise, the preponderance of evidence suggests that resistance training may reduce the risk of fracture not only by improving bone parameters but also by preventing falls in older adults. Therefore, resistance training that induces high levels of mechanical strain by making muscles work against a weight or force is expected to prevent age-related bone loss and falls in those already diagnosed with osteoporosis or osteopenia, and it should be recommended for those populations.[48]
The guideline also recommended the impact exercise for at least 6 months with 3 or more days per week, and start at ten jumps and progressing to 50 jumps per session (I/A). For example, Australian guidelines recommended that impact exercise with 50 jumps per set for 3 to 5 sets and 10 to 20 repetitions with 1 to 2 min rest between sets for 4 to 7 days per week would improve bone health.[20] They recommended high-intensity (>4 times body weight) impact exercise for those without osteoporosis but 2 to 4 times body weight for those at moderate risk of osteoporosis. According to a recent consensus from the UK Expert Exercise Steering Group (EESG), moderate- to high-intensity impact exercise including jumping, hopping, running, and Scottish dance or Zumba with a minimum of 50 impacts per session while avoiding landing from a height.[64] Likewise, our guideline recommended that individuals with osteoporosis should undertake resistance training and impact exercise to maximize bone strength. In practice, all muscle groups, including the back muscles, should be targeted to improve bone strength in the spine. Also, the patients with osteoporotic conditions are required to start with low-intensity exercises ensuring good technique and a maximum of 8 to 12 lifts to build up to 3 sets for each exercise.[63] Furthermore, the combination of progressive resistance and impact exercise best promotes bone strength based on evidence similar to the other national guidance.[63]
Our guidelines also proposed an intermediate level of evidence and less strong recommendations for weight-bearing aerobic exercise, PA, and balance training in individuals with osteoporosis. Balance training should include several exercise stimuli, including dynamic and static balance with gradual increases in the intensity, such as tandem foot standing, multi-directional weight lifts, heel-toe walking, line walking, standing on one leg for at least 6 months with 1 to 3 or more day per week for more than 15 min per day (II /B). Although less strongly recommended, weight-bearing aerobic exercise more than 3 times per week, with 20 or more min per day (II /B), could be more welcoming for many people than resistance exercise since it does not necessarily require special facilities or equipment. The ACSM suggests that carrying out simple weight-bearing endurance activities, such as stair climbing, jogging, and tennis, which include motion with sufficient gravitational or mechanical load and possibly in combination with other types of exercise during adulthood, are recommended for bone health.[32] Our guideline recommended that the patients with osteoporosis perform the weight-bearing exercise intensity at least in RPE of 11 to 14 on the Borg scale, an alternative method for prescribing exercise intensity, or 40% to 60% of heart rate reserve or 3 to 6 metabolic equivalents to generate positive outcomes. Moreover, the proper frequency, intensity, and time are dependent on the type of PA (Tai chi, dynamic flamingo, line dance, foot stamps, etc.), but PA at least 2 to 5 days a week, 10 to 60 min a day for more than 6 months is recommended (II/B). The SIOMMS guides recommend performing a minimum of PA, such as walking, for 30 min every day, despite the lack of available evidence.[65] PA such as Tai Chi and dance may be of benefit to individuals with low bone density because of their positive effect on fall risk and postural control, suggesting that in combination with its modest effects of BMD, PA is a potentially valuable intervention for prevention of falls and fall-related fractures in postmenopausal osteopenic women.[60,61] The consensus was that PA that improve balance and muscle strength, including Tai Chi, dance, and yoga could be conducted, at least twice a week in line with PA guidance.[64]
Although the effect of exercise on BMD is modest, a meta-analysis concluded that improvement in the lumbar spine and femoral neck BMD with exercise reduces the risk of osteoporotic fractures by approximately 10%.[66] Additionally, since about 95% of hip fractures are caused by falls, fall prevention by improving postural balance may be more important than the effect of exercise on BMD in terms of fracture. As impaired balance was a significant contributor to falls in the older generation,[67] the enhanced postural balance could reduce the risk of fractures by improving postural stability and mobility and diminishing the risk of falls.[68] Balance training was revealed to effectively prevent falls and improve the quality of life in the older adults.[45, 69] The most effective fall-prevention exercise programs have yet to be clarified, but programs targeting strength and balance appear particularly effective.[65] Strength and balance training is recommended that is individualized, supervised by an exercise professional, and conducted for 3 hr per week over at least 4 months, in line with previous evidence.[70] A combination of balance exercises and other weight-bearing exercises may also be necessary to prevent age-related bone loss and falls in older individuals with low bone mass. This guideline recommended strength and balance training to reduce fall incidence and enhance physical function based on evidence. Previous studies found that a short duration of resistance and balance training improved strength, balance function, and fear of falling, which may reduce fall risk and increase confidence to remain active.[71] However, considering that older people have been diagnosed with osteoporosis or osteopenia, it is also effective to apply an additional load on light PA to eliminate the possibility of fractures accompanied by exercise in terms of safety. For this reason, several studies have recently published alternatives that have similar effects of exercise, such as whole-body vibration (WBV). In general, WBV in multiple settings showed increased lumbar spinal and femoral BMD and improved bone remodeling activities in the osteoporosis and osteopenia population.[40,72,73] However, it is complex to define WBV as a PA that can replace exercise.
The schematic image of the exercise guidelines to improve osteoporosis and prevention of fall is shown in Figure 2.
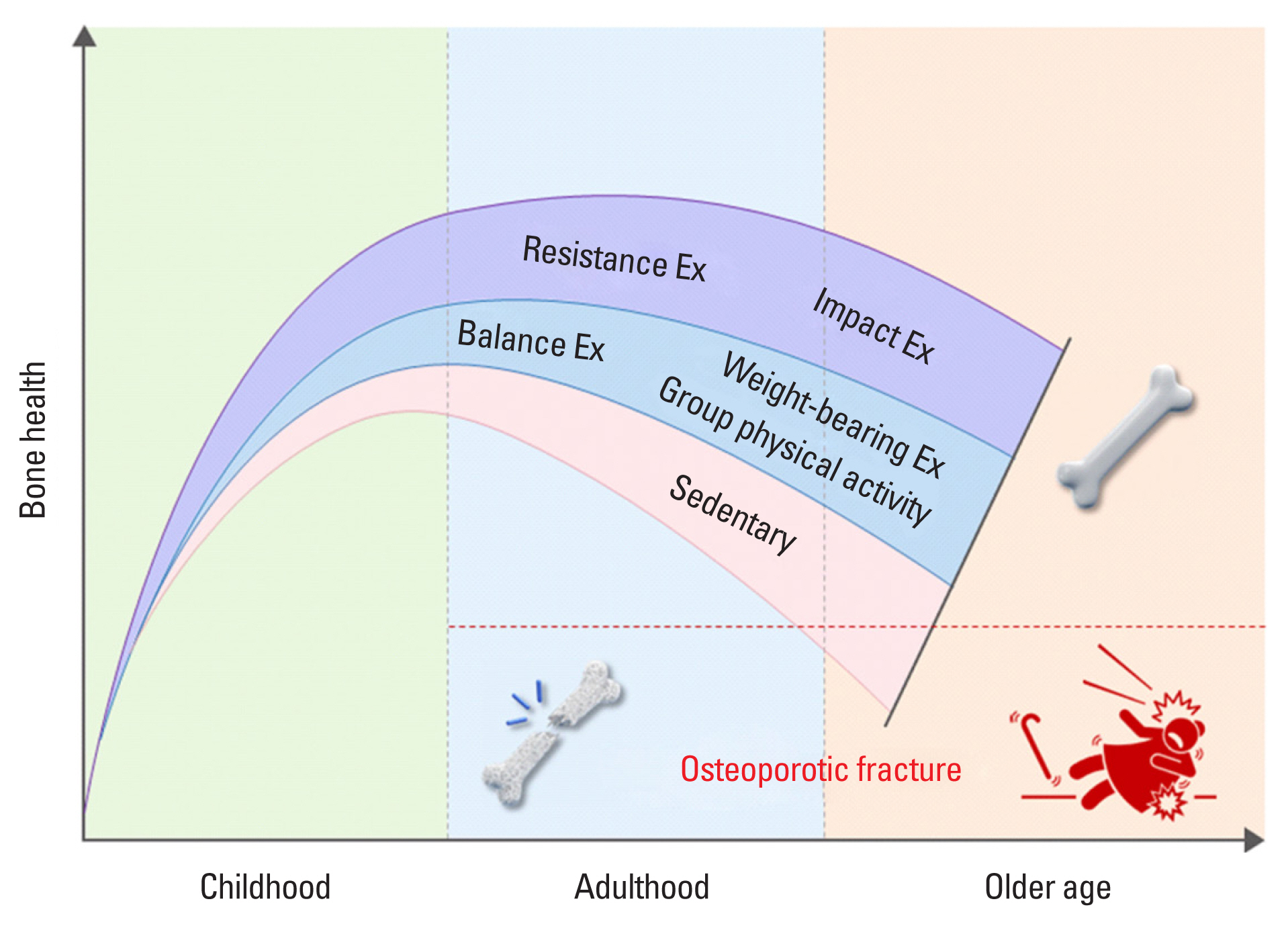
Exercise guidelines to improve osteoporosis and prevention of fall for individuals with osteoporosis or osteopenia.
The key recommendations in this guideline are that individuals with osteoporosis or osteopenia should undertake resistance and impact exercise to maximize bone strength. It is recommended that resistance training should be performed 2 or 3 times a week, with one set of 5 to 12 repetitions by a combination of free weights and machines, including 3 to 10 exercises in major muscle groups at 50% to 85% 1RM for 3 to 12 months. Impact exercise is recommended to include jump drop, jumping chin-ups with drop landings, and jump rope with 50 jumps per session for at least 6 months, with 3 or more days per week. Moreover, weight-bearing aerobic exercise, balance training, and habitual PA prevent further bone loss, maintain or improve BMD and reduce the risk of falls in the individual with osteoporosis and osteopenia. While it is evident that resistance and impact exercise or combinations of those 2 exercise programs influence both BMD and fracture prevention, it is believed that exercise prescriptions must fully consider the individual’s health status and characteristics.
Supplementary Information
Notes
Ethics approval and consent to participate
Not applicable.
Conflict of interest
No potential conflict of interest relevant to this article was reported.