Bone Mineral Density in Patients with Pediatric Inflammatory Bowel Disease Using Dual Energy X-Ray Absorptiometry
Article information
Abstract
Background
Inflammatory bowel disease (IBD) is a chronic inflammatory immune-mediated condition that affects the gastrointestinal system and alters bone growth and bone mineral density (BMD). Here we aimed to study the prevalence and predictors of a low BMD in pediatric patients with IBD.
Methods
This retrospective cross-sectional analytical study included pediatric patients with IBD in whom BMD was evaluated using dual energy X-ray absorptiometry of the total body and lumbar spine. Osteoporosis was defined as a BMD Z-score ≤−2, osteopenia as −2 to −1, and normal as >−1. Clinical and laboratory findings were compared between patients with and without osteoporosis.
Results
Of the 48 patients, 30 (62.5%) were males, 35 (72.9%) had Crohn’s disease, and 13 (27.1%) had ulcerative colitis. The mean age at diagnosis was 9.9±2.8 years. The median age at the time of the BMD scans was 11.9 (interquartile range, 9.9–14.3) years. Total body BMD scans identified 13 (27.1%) and 16 (33.3%) patients with osteoporosis and osteopenia, respectively. Spinal BMD scans revealed that 17 (39.5%) and 14 (32.6%) patients had osteoporosis and osteopenia, respectively. A low body mass index (BMI) Z-score (P=0.038), ileocolonic disease location (P=0.008), and a low calcium level (P=0.008) were significant predictors of osteoporosis on the total body BMD scans. A low BMI Z-score (P=0.039), decreased hemoglobin level (P=0.018), low calcium level (P=0.033), and infliximab use (P=0.019) were significant predictors of osteoporosis on the spinal BMD scans.
Conclusions
This study showed a high prevalence of low BMD among pediatric patients with IBD. A low BMI, ileocolonic disease location, low hemoglobin and calcium levels, and infliximab use were significantly associated with osteoporosis.
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic inflammatory condition that primarily affects the gastrointestinal (GI) tract and is further classified into ulcerative colitis (UC) and Crohn’s disease (CD).[1] Although an exact etiology is lacking, IBD is considered an immune-mediated disease that causes dysregulation between the core of the immune system and the innate and adaptive immune responses that attack the intestinal luminal bacteria.[2,3] In addition to the immunological factors, the disease’s pathogenesis can be determined by environmental factors in a genetically susceptible individual as well.[2,3] IBD usually manifests as abdominal pain, diarrhea, and rectal bleeding.[3] Moreover, it has multiple extraintestinal symptoms, including skeletal, dermatological (erythema nodosum and pyoderma gangrenous), ocular (episcleritis and uveitis), and hepatobiliary (primary sclerosing cholangitis).[3] The pulmonary and renal systems can also be involved but to a lesser extent.[4]
Childhood and adolescence are considered critical periods for bone growth and development with which an inflammatory process (such as IBD) will interfere with bone growth and density.[5–7] Multiple factors can lead to altered bone growth in patients with IBD, including chronic inflammation, inflammatory cytokine release, medication effects such as steroids, poor nutrition, intestinal malabsorption, decreased vitamin D, and decreased physical activity.[5–8]
The bone mineral density (BMD) test provides an overall evaluation and estimation of a patient’s bone health, risk of fractures, and diagnosis of osteoporosis.[9] One of the most widely used methods for assessing BMD is the central dual energy X-ray absorptiometry (DXA) test, which measures bone density in the hip and lumbar spine.[9] Interpretation of results is represented as a Z-score with normal values of +1, 0, and −1, while values of less than −1 are considered low bone mass density with a higher predisposition to fractures.[9]
Several studies from neighboring countries have reported the effects of IBD on BMD. However, no studies have described the impact of pediatric IBD on BMD in the Kingdom of Bahrain. We hypothesized that IBD would negatively impact BMD in pediatric patients. Therefore, this study aimed to measure the prevalence of impaired BMD in pediatric IBD patients in the Kingdom of Bahrain and to identify the clinical and laboratory predictors of a decreased BMD.
METHODS
1. Study design and setting
This retrospective cross-sectional analytical study included patients with IBD attending the pediatric department at Salmaniya Medical Complex in the Kingdom of Bahrain between April 2013 and September 2022.
2. Study population
This study included pediatric patients aged ≤18 years with IBD who underwent DXA for a BMD assessment. Patients with primary bone disease, with comorbidities leading to bone fragility, and who did not undergo a DXA assessment were excluded. The electronic National Health Information System (I-SHEA) medical registry of the Salmaniya Medical Complex was used to conduct this study. The diagnosis of IBD was based on clinical, radiological, endoscopic, histopathological, and biochemical results, which were reviewed using European Society of Pediatric Gastroenterology, Hepatology, and Nutrition criteria and North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition criteria.[10,11] Accordingly, children and adolescents were suspected to have IBD if they presented with symptoms such as abdominal pain, diarrhea, rectal bleeding, and weight loss that persisted for >4 weeks or recurrent for >2 episodes within 6 months. These patients were referred to a pediatric gastroenterologist for diagnostic confirmation. After excluding infectious causes and performing laboratory screening tests, patients underwent endoscopy and biopsy. The combination of clinical symptoms and endoscopic and histologic findings will enable a diagnosis of IBD-type CD or UC. Apart from definite UC, radiological evaluation of the small bowel is mandatory.[10]
3. Data collection
The I-SEHA system was utilized to record and review data on sex, age at diagnosis and presentation, nationality, initial clinical presentation, family history of IBD, extraintestinal manifestations, medications used for treatment, and surgical interventions related to IBD. The location and behavior of CD and the extent of UC were classified according to the Paris classification.[12] The localization of the disease includes L1 (terminal ileum), L2 (colonic), L3 (ileocolonic), L4a (upper GI disease, proximal to the ligament of Treitz), and L4b (upper GI disease between the ligament of Treitz and the distal 1/3 of the ileum), and disease behavior including B1 (non-structuring and non-penetrating), B2 (structuring), and B3 (penetrating). The disease extent for UC included E1 (ulcerative proctitis), E2 (left-sided UC distal to the hepatic flexure), E3 (extensive; hepatic flexure distally), and E4 (pancolitis; proximal to distal hepatic flexure). Laboratory data including complete blood count, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), liver function test, anti-Saccharomyces cerevisiae antibodies (ASCA), antineutrophil cytoplasmic antibodies (ANCA), 25-hydroxy-vitamin D (25[OH]D), calcium, and phosphate were obtained and reviewed.
4. BMD measurement
BMD was evaluated using DXA scans of the body and spine (Hologic QDR 4500; Hologic Inc., Bedford, MA, USA). Total body and anteroposterior (AP) lumbar spine (L1–4) measurements and BMD Z-scores were calculated at the Radiology Department, Salmaniya Medical Complex. These parameters are considered valuable for evaluating BMD in children.[6,13] The International Society for Clinical Densitometry guidelines define osteoporosis as a BMD Z-score ≤−2 standard deviation (SD), osteopenia as a Z-score between −2 and −1 SD, and normal BMD as a Z-score > −1 to +1 SD.[14]
5. Anthropometric measurements
Weight was measured using a digital weighing scale and the results were interpreted in kilograms (kg). Height is expressed in centimeters (cm) and was measured using a standard wall-bound stadiometer. Patient weight and height were measured during the DXA scan and converted to SD Z-scores in addition to the total body mass index (BMI) using an anthropometric software program EpiInfo® (Centers for Disease Control and Prevention, Atlanta, GA, USA).
6. Statistical analysis
Patient information and data were first collected and recorded on an Excel sheet and then analyzed using the SPSS Statistics version 21 (SPSS Inc., Chicago, IL, USA). Demographic data were expressed as frequencies and percentages. Numerical data were presented as mean and SD for normally distributed variables and median and interquartile range (IQR) for non-normally distributed variables. Patients were divided into 2 groups based on the BMD results (osteoporotic [<−2 SD] and non-osteoporotic [≥−2 SD] for total body and spinal BMD). To detect the predictors of osteoporosis, the clinical characteristics and laboratory findings of both groups were compared using univariate analysis. IBD location was further categorized according to the area of major involvement (small bowel, ileocolonic, and colonic). A one-way analysis of variance (ANOVA) test was conducted to compare disease locations in terms of the mean total body BMD Z-scores. If the result indicated a significant difference, a post-hoc power analysis was conducted using the least significant difference (LSD) and Bonferroni post-hoc tests. The confidence interval was set at 95%. A P-value of less than 0.05 was considered statistically significant.
7. Ethical consideration
The study was approved by the Secondary Care Medical Research Subcommittee at Salmaniya Medical Complex, Ministry of Health, Kingdom of Bahrain (no. 76120521). The ethical principles of the Declaration of Helsinki were followed for this study. Written informed consent was obtained from the patients’ parents or guardians.
RESULTS
During the study period, 152 patients with pediatric IBD were registered. A total of 105 patients were excluded from the study (103 did not undergo a BMD scan, one patient’s parents refused the procedure, and one patient moved a lot during the procedure, so it was aborted). The remaining 48 (31.6%) patients who underwent BMD scan evaluation were included. The demographic and clinical characteristics of the included patients are presented in Table 1. The mean age at diagnosis was 9.9±2.8 years. The median age at the BMD scan was 11.9 (IQR, 9.9–14.3) years, and 62.5% of patients were males. Thirty-five (72.9%) patients had CD and 13 (27.1%) had UC.
The results of laboratory investigations of the study population are presented in Table 2. Abnormal results were as follows: leukocytosis (N=19, 41.3%), leukopenia (N=2, 4.3%), anemia (N=32, 69.5%), thrombocytosis (N=28, 60.9%), hypoproteinemia (N=4, 9.1%), hypoalbuminemia (N=29, 66%), hypocalcemia (N=9, 22%), hypercalcemia (N=3, 7.3%), hyperphosphatemia (N=3, 7.9%), low 25(OH)D (N=29, 72.5%), high CRP levels (N=31, 68.9%), and high ESR (N=28, 60.9%). ANCA was positive in one of the 12 tested patients, while ASCA was positive in 2 of the 3 tested patients.
The medical therapies administered to patients are presented in Table 3. Prednisolone was the most commonly prescribed medication which was received by 22 (45.8%) patients, 5 (10.4%) of which were steroid-dependent. Biological therapy was used in 17 (35.4%) patients, 15 of whom received adalimumab and 2 received infliximab.
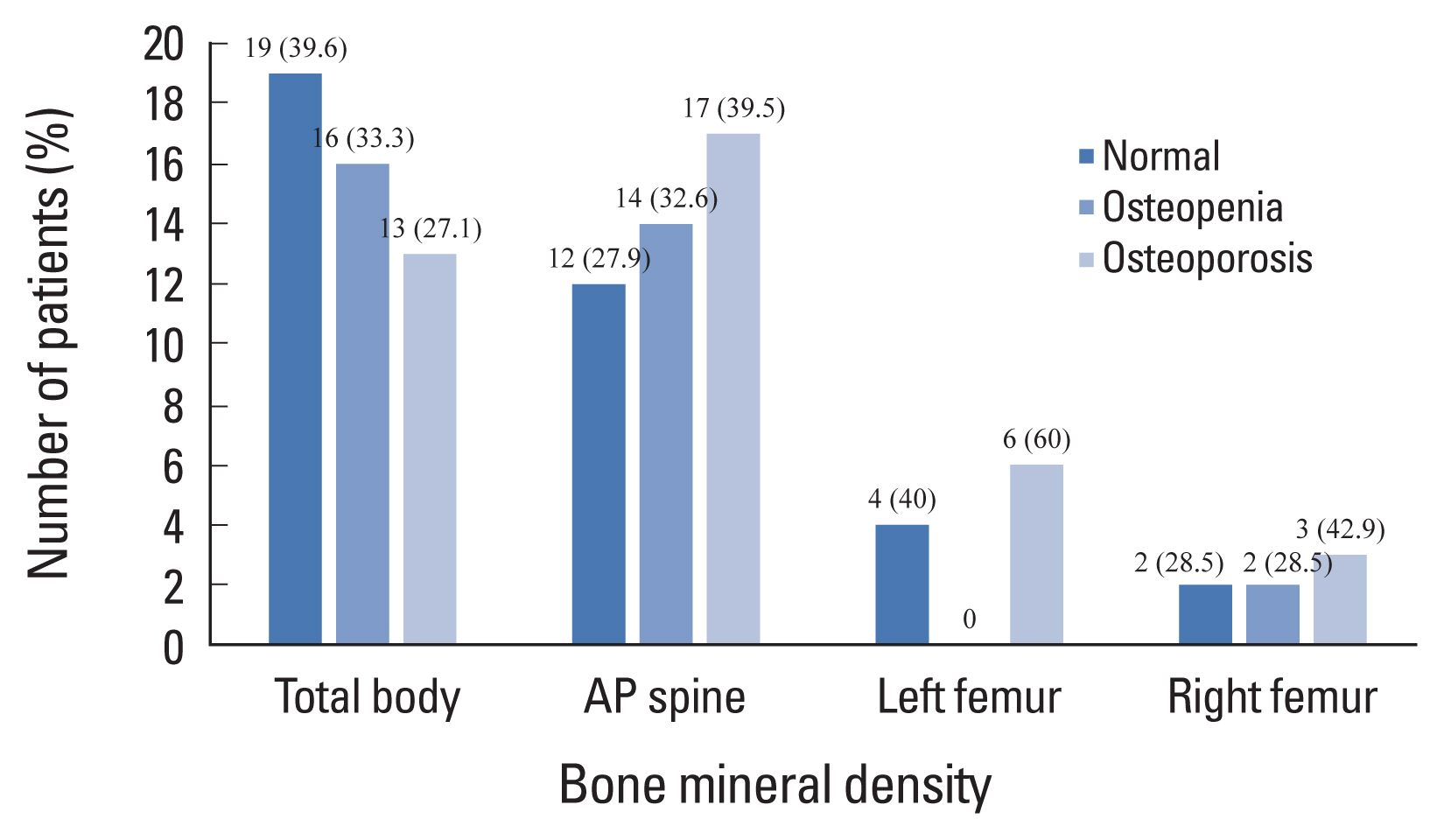
The results of BMD scans are shown in Figure 1. All 48 patients underwent total body BMD scans: 43 (89.6%) had AP spine BMD scans, 10 (20.8%) had left femur BMD scans, and seven (14.6%) had right femur BMD scans. Osteoporosis was found in 13 (27.1%), 17 (39.5%), 6 (60%), and 3 (42.9%) patients.
Comparisons between osteoporotic (Z-score ≤−2) and non-osteoporotic (Z-score >−2) patients based on total body and AP spine BMD scans are shown in Table 4. Total body BMD scans showed that osteoporotic patients had significantly lower BMI Z-scores (P=0.038), ileocolonic disease location (P=0.008), and lower calcium (P=0.008) levels than non-osteoporotic patients. Non-osteoporotic patients had a statistically significant tendency to have colonic disease location (P=0.018). AP spine BMD scans also revealed that osteoporosis was significantly associated with lower BMI Z-scores (P=0.039), calcium levels (P=0.033), and hemoglobin levels (P=0.018), but with higher infliximab use (P=0.019). In both total body and AP spine BMD scans, there were no significant differences found in relation to age at diagnosis, age at BMD scan, follow-up duration, sex, type of IBD, weight and height Z-scores, disease behavior, presence of perianal disease, extraintestinal manifestations, white blood cell count, serum protein, albumin, alkaline phosphatase, phosphorus, vitamin D level, CRP, ESR, and the use of prednisolone, azathioprine, mesalamine, or vitamin D supplementation.
On comparing the 3 types of disease locations in terms of the mean total body BMD Z-scores, a one-way ANOVA test indicated a significant difference in the total BMD scores among the 3 different disease locations, F (2, 34)=4.326, P=0.021. Subsequently, post-hoc power analysis was conducted using LSD and Bonferroni post-hoc tests. Pairwise comparisons using the LSD test revealed a significant difference between small bowel and colonic involvement (P= 0.017) but not with ileocolonic disease (P=0.058). More specifically, the mean BMD Z-scores in patients with small bowel involvement (−3.2±1.1) were significantly lower than in patients with colonic disease (−0.9±1.1). However, there was no significant difference between patients with small bowel diseases and those with ileocolonic involvement (−1.7±1.4; P=0.126). The difference between small bowel and colonic disease was not significant according to the Bonferroni test result (P=0.050), which might indicate the presence of a type I error.
DISCUSSION
This study showed that the prevalence of osteoporosis in patients with pediatric IBD using total body and AP spine BMD scans was 27.1% (N=13/48) and 39.5% (N=17/43), respectively, whereas that of osteopenia was 32.6% (N= 14/43) and 33.3% (N=16/48), respectively. Although the prevalence of osteoporosis and osteopenia in Bahrain has been well documented in adults, this is not the case in the pediatric age group, which makes comparisons difficult. The first retrospective study conducted by Sadat-Ali and Mattar [15] found that of 205 adults enrolled patients, 79 (38.5%) were osteoporotic and 97 (47.3%) were osteopenic. However, in a recent cross-sectional study of young females (mean age, 47±11 years) in Bahrain, Alsayyad et al.[16] found that, of 892 females, 4.9% were osteoporotic and 29% were osteopenic. The latter values were lower than those of patients with pediatric IBD. IBD is strongly associated with a reduced BMD in several geographical locations in the region and worldwide.[17] Reports are in line with the fact that bone health in patients with pediatric IBD, particularly those with CD, has a high rate of a low BMD. Saadah et al.[13] in Saudi Arabia revealed a 39% prevalence of osteoporosis on both total body and AP spine scans, whereas the prevalence of osteopenia using total body BMD and AP spine scans was 31.3% and 28.1%, respectively. These findings are comparable to those of our study, although their study included only patients with CD.[13] However, Guz-Mark et al.[6] reported a prevalence of 8.2% for osteoporosis and 44.3% for osteopenia in pediatric patients with IBD in the Middle East. Moreover, other studies of osteoporosis in several other regions revealed a prevalence of 4% in France using total body scans, 26.7% in Sweden using AP spine scans, and 27.5% and 12.5% in the USA using both total body and AP spinal scans, respectively.[7,18,19] The prevalence of osteopenia is 51% in France, 46.7% in Sweden, and 31% and 33.3% in the USA based on total body and AP spine scans, respectively.[7,18,19]
In the current study, children with a lower BMI had more osteoporosis in total body and spinal BMD studies (P=0.038 and P=0.039, respectively). Saadah et al.[13] found that low weight and height Z-scores were associated with the development of osteoporosis in patients with CD, yet low BMI Z-scores were not significantly associated with a low BMD. However, contrary to our findings, Setty-Shah et al.[20] concluded that the AP spine BMD Z-score was much lower in obese IBD patients with high BMI levels than in controls. Additionally, Wada et al.[21] observed that IBD patients with a high BMI had low BMD levels.
The relationship between age at the time of the IBD diagnosis and the results of BMD scans remains an area of debate. Our findings show no significant association between these 2 factors. This coincides with the findings of Levy-Shraga et al.[22] and Lopes et al.[23], in which there was no association between age at the time of diagnosis and a low BMD. This is in contrast to the findings of Saadah et al.[13], Sohn et al.[24], and Herzog et al.[25], who reported that older age was strongly associated with lower BMD Z-scores in pediatric patients diagnosed with IBD.
In this study, children with IBD involving the ileocolonic area were more likely to have osteoporosis on the total body BMD, while those with colonic disease alone were less likely to have osteoporosis on the total body BMD (P=0.008). This confirms the vital role of the ileocolonic area in the absorption of electrolytes and minerals. The inflamed mucosa of patients with IBD shows impaired absorption of these electrolytes.
Vitamin D also plays a role in maintaining calcium homeostasis and bone metabolism; it is converted by the liver and kidneys into its active metabolites.[24,26] The active form of vitamin D (25[OH]D) aids in the absorption of calcium from the GI tract as well as the reabsorption of calcium and phosphate from the kidneys, an essential process for bone mineralization.[24,26] In our study, low serum 25(OH)D levels contributed to osteoporosis in patients with IBD, but this difference was not statistically significant. This is supported by 2 studies conducted in pediatric patients with IBD, in which significantly low vitamin D levels predicted low BMD.[13,27] In contrast, other studies found no association between vitamin D levels and a low BMD.[24, 28,29] Individuals with IBD have a higher risk of vitamin D deficiency due to poor oral intake and impaired absorption of vitamin D secondary to the inflammatory process of their disease, especially during flare-ups.[24]
Osteoporosis in patients with IBD has been linked to dietary calcium deficiency, abnormal absorption in the gut, and glucocorticoid use.[30] Osteoporosis based on total body and spinal BMD was significantly associated with lower serum calcium levels (P=0.008 and P=0.033, respectively) in our study, which was in accordance with Sohn et al. [24] biochemical profiles of children with IBD. However, other studies found no significant difference in serum calcium, phosphate, albumin, and creatinine levels in patients with low BMD.[27,31]
This study found that AP spine BMD was negatively affected by low hemoglobin levels, as children with lower hemoglobin levels had more osteoporosis in spinal BMD (P=0.018). Sohn et al.[24] observed similar results of a positive correlation between hemoglobin levels and BMD in children with CD. Many causes play a role in the development of anemia in IBD patients, including nutritional iron deficiency, chronic inflammation of the mucosa leading to impaired iron absorption, and blood loss due to mucosal ulcerations.[32]
Infliximab is a tumor necrosis factor-α antagonist that contributes to increased bone formation and decreased bone resorption at the cellular level.[33] CD patients undergoing biological treatment (infliximab or adalimumab) have better control and maintenance against CD symptoms and flare-ups and a decreased risk of bone resorption, osteoporosis, and long-term corticosteroid complications.[34] In our study, children with osteoporosis in terms of spinal BMD more often required infliximab than those without osteoporosis (P=0.019). This can be attributed to the fact that these patients had already developed a severe form of IBD that warranted the initiation of biological treatment. This finding suggests that osteoporosis occurs more frequently in children with severe IBD who require biological therapy.
This study was limited by its small sample size. This was expected, as not all parents/guardians agreed to expose their children to radiation. Nonetheless, despite being small, our sample was considered representative since it compares the total number of patients with IBD to the number of study participants. In addition, data extraction from a single hospital in Bahrain may not be representative. The results of this study are not from a comparative study with the general population, and it is inappropriate to generalize its findings, including risk factors, to the general population. In Bahrain, only 2 adult studies tackled osteoporosis and osteopenia, but there were no reports about these conditions in the pediatric age group for comparison.[15,16] However, our institution is considered the main tertiary hospital that serves most of the population in the Kingdom of Bahrain. It is the only hospital providing care for pediatric patients with IBD and has the largest patient database, making it representative of this group of patients. Another limitation of this study was that the appointment for BMD was not given and not performed immediately upon the time of diagnosis. Indeed, it was administered a few months after diagnosis in some patients.
Despite these limitations, our study is the first conducted in the Kingdom of Bahrain to investigate the effects of IBD on the BMD of pediatric patients. This study is unique in that it reports data on osteopenia along with osteoporosis, which has not been reported in many studies. The findings of this study emphasize the importance of early screening for low BMD and the detection of associated risk factors in patients with pediatric IBD. Moreover, the results of this study can help the caring physician to intervene at an earlier stage to prevent the further progression of osteopenia to osteoporosis and to treat those affected to avoid long-term complications. Furthermore, there are very few similar studies in the region, which makes the findings of this study important for future studies and applicable to meta-analyses.
CONCLUSION
This study showed a high prevalence of osteoporosis and osteopenia among pediatric patients with IBD. A low BMI Z-score, ileocolonic disease location, low hemoglobin, low calcium, and infliximab use were significantly associated with osteoporosis when measured using total body or AP spine BMD scans. Further studies are needed to explore the different mechanisms of osteoporosis and osteopenia in children with IBD and their responses to medical therapies.
Acknowledgments
The authors gratefully acknowledge the doctors and nurses involved in the care of pediatric patients with inflammatory bowel diseases in the pediatric and radiology departments of our institution.
Notes
Ethics approval and consent to participate
The study protocol conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board of the Secondary Care Medical Research Subcommittee at Salmaniya Medical Complex, Ministry of Health, Kingdom of Bahrain (no. 76120521).
Conflict of interest
No potential conflict of interest relevant to this article was reported.