Position Statement: Vitamin D Intake to Prevent Osteoporosis and Fracture in Adults
Article information
Abstract
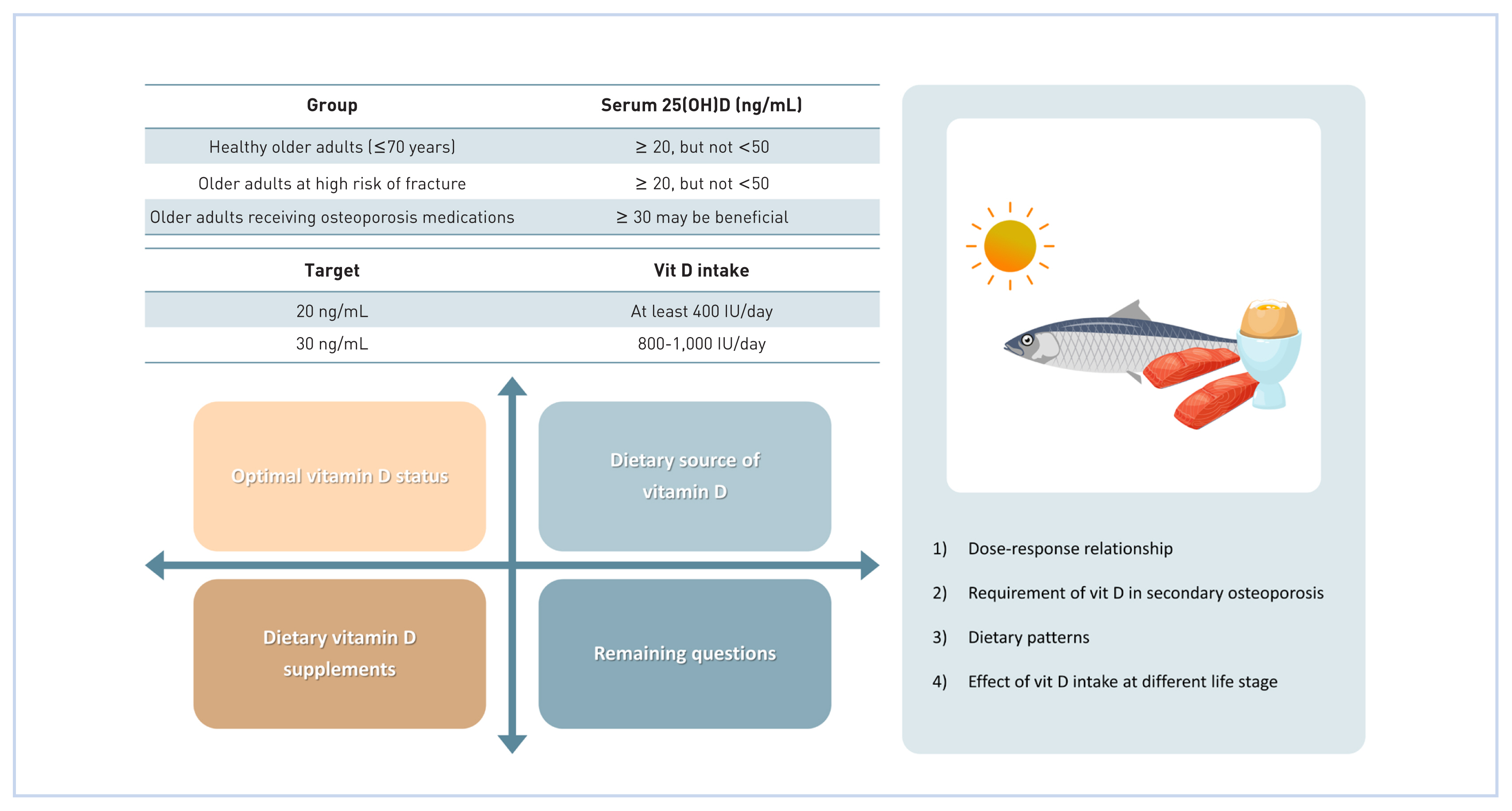
Adequate vitamin D status is essential for bone health. New randomized controlled trials investigating the effect of vitamin D supplementation on bone health have recently been published. This position statement updates and expands on the previous 2015 position statement of the Korean Society for Bone and Mineral Research on the adequate vitamin D status for healthy older adults (age ≥ 70 years) and those at high risk of osteoporosis and fracture (adults on osteoporosis medications) to maintain serum 25-hydroxy-vitamin D (25[OH]D) levels ≥ 20 ng/mL but < 50 ng/mL. A serum 25(OH)D level of 30 ng/mL may be beneficial for those on anti-resorptives. Vitamin D can be obtained from ultraviolet light exposure and diet. To reach the target vitamin D status through intake, adults must consume at least 400 IU/day to reach 20 ng/mL and 800 to 1,000 IU/day to reach 30 ng/mL. Foods familiar to the Korean diet that are high in vitamin D content or consumed frequently enough to positively impact vitamin D status are introduced in addition to the amount required to help reach one’s target vitamin D status.
INTRODUCTION
Osteoporosis is a common systematic bone disease identified by reduced bone mineral density (BMD), bone quality, and strength; it increases the risk of fractures, reduces the quality of life, and increases the risk of mortality.[1] Currently, approximately more than 200 million people are diagnosed with osteoporosis and this number is expected to increase due to the global trend of increase in the aging population.[2,3] Approximately 48% of older Korean adults (≥ 50 years) have osteopenia and 22% have osteoporosis.[4,5] The incidence of osteoaporotic fractures (Caese/10,000 person-years) steadily increased until 2013 and has plateaued since; however, the total number of cases osteoporotic fractures have gradually increased since 2008.[4,5] As a result, total medical costs for osteoporotic patients increased from 1,864 million South Korean Won (KRW; 2013) to 2,827 million KRW (2017) with an annual increase of 11%.[6] Although multiple osteoporosis medications are available, the risk of severe side effects limits the long-term use of each treatment and reduces patients’ adherence to medications.[7] Therefore, the prevention and effective treatment of osteoporosis is critical.
Vitamin D plays an essential role in the regulation of bone and mineral metabolism. In the endocrine system, vitamin D is obtained from cutneous synthesis and food consumption. Vitamin D is metabolized to 25-hydroxy-vitamin D (25[OH]D) via hydroxylation in the liver, which is then metabolized to 1,25-dihydroxy-vitamin D (1,25[OH]2D), the biologically active form of vitamin D, in the kidney (Fig. 1).[8,9] As serum 25(OH)D is the most abundant circulating vitamin D metabolite with a relatively long half-life (2–3 weeks),[10] it is currently utilized as the nutritional status marker of vitamin D.[11–13] Serum 1,25(OH)2D binds to the vitamin D receptor (VDR), which forms a heterodimer with the retinoid X receptor (RXR). The VDR-RXR complex translocates to the nucleus and binds to vitamin D response elements, regulating gene transcription involved in intestinal absorption, bone resorption, and renal reabsorption of calcium and phosphorus.[14,15] Thus, vitamin D deficiency results in the impairment of calcium absorption and dysfunction of bone remodeling, leading to the reduction of BMD, fragile bones, and the elevation of fracture risk.[16,17] Locally, 1,25(OH)2D directly controls osteoblastogenesis and osteoclast differentiation, while its distinct involvements in osteocytes are yet unclear.[18] Koreans have relatively low vitamin D intake [19] and vitamin D status [20] which may increase the risk of osteoporosis and fractures.
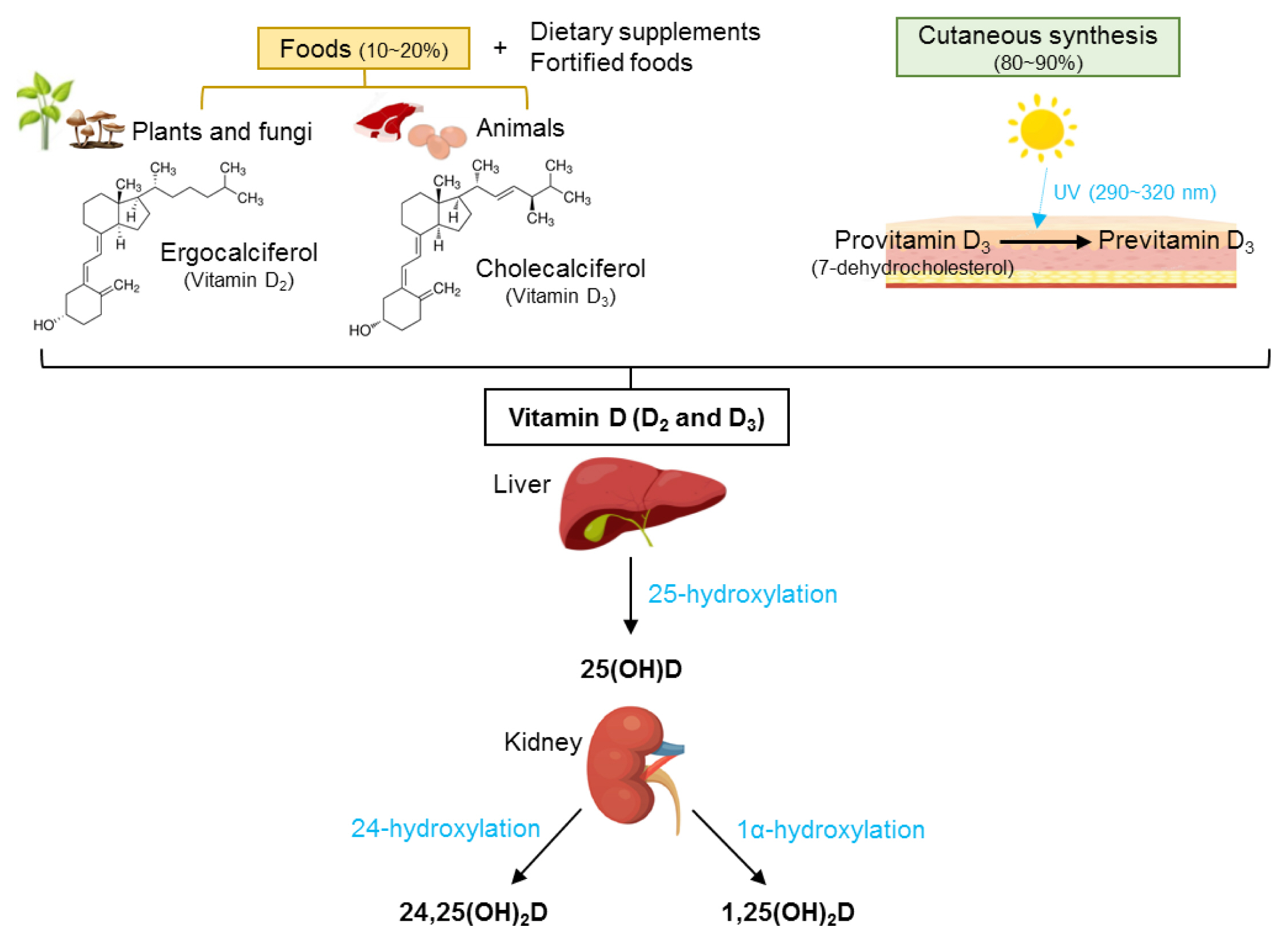
Metabolism of vitamin D. 25(OH)D, 25-hydroxy-vitamin D; 24,25(OH)2D, 24,25-dihydroxy-vitamin D; 1,25(OH)2D, 1,25-dihydroxy-vitamin D.
In 2015, the Korean Society for Bone and Mineral Research published a position statement on adequate intake levels of calcium and vitamin D in older men (≥50 years) and postmenopausal women, with a focus on calcium and vitamin D supplements.[21] The current position statement updates the previous statement regarding vitamin D intake with recent evidence on optimal vitamin D status in healthy older adults, older adults with high fracture risk, and older adults receiving osteoporosis medications in Korea. In addition, the present statement introduces dietary sources of vitamin D for Koreans to achieve optimal vitamin D status to maintain optimal skeletal health.
OPTIMAL VITAMIN D STATUS FOR BONE HEALTH
Low vitamin D status is associated with inadequate bone mass or remodeling, causing elevated fracture risk.[15,22] However, the optimal vitamin D status for bone health is still controversial among organizations.[11,23,24] Therefore, we hereby summarize the most recent scientific evidence to recommend an optimal vitamin D status for the bone health of adults.
1. Healthy older adults (<70 years of age)
Previously, most vitamin D supplementation trials on BMD were co-supplemented with calcium, obscuring the effect of vitamin D alone, or were secondary analyses resulting in a lack of power to demonstrate the effect of vitamin D on the bone which is the potential reason for the inconsistent results.[25–27] In 2011, the Institute of Medicine (now the National Academy of Medicine) established the target serum 25(OH)D levels for the Recommended Daily Allowance as 20 ng/mL (50 nmol/L) to meet the needs of 97.5% of the healthy North American population and use as an individual’s nutritional goals.[23] More recent studies show results supporting the recommendations. For instance, a meta-analysis reported the possibility that vitamin D supplementation of 800 IU/day (20 μg/day) improves BMD in older adults who have serum 25(OH)D less than 20 ng/mL. [28] In a pooled analysis, older adults with baseline serum 25(OH)D ≥17.2 ng/mL had a lower risk of nonvertebral fracture compared to those with low baseline serum 25(OH)D (<12 ng/mL),[29] although the number of studies with baseline serum 25(OH)D data was few. Recent well-designed randomized controlled trials (RCTs) failed to demonstrate the advantageous effects of vitamin D supplementation on BMD in healthy individuals with a mean vitamin D status ≥20 ng/mL from earlier observational studies. Daily supplementation of 2,000 IU (50 μg/day) vitamin D for 2 years increases serum 25(OH)D from 27.6 to 39.4 ng/mL but did not improve BMD.[30] Surprisingly, supplementing vitamin D in older adults with mean baseline serum 25(OH)D above 30 ng/mL with 4,000 or 10,000 IU/day (100 or 250 μg/day) for 3 years led to the elevation of serum 25(OH)D to 52.9 or 57.8 ng/mL, respectively, but decreased BMD dose-dependently,[31] especially in women [32], compared to those with relatively lower vitamin D status (31 ng/mL). The additional sub-analyses of these studies in adults with lower vitamin D (<20 ng/mL) and secondary and meta-analyses of previous studies indicate that adults with serum 25(OH)D <20 ng/mL may benefit from increasing vitamin D status.[25,30]
In conclusion, these findings suggest that serum 25(OH)D >20 ng/mL is adequate for healthy older adults. However, serum 25(OH)D ≥50 ng/mL may not be beneficial for bone in healthy older adults.
2. Older adults (≥70 years of age) at a high risk of fracture
Vitamin D supplementation (with or without calcium supplementation) has been recommended for osteoporosis patients to delay the progression of osteoporosis and prevent the incidence of osteoporotic fractures.[1] However, the optimal vitamin D status for high-risk patients, such as adults ≥70 years of age have not been established.
Adequate vitamin D status is required for elderly adults (≥70 years) since this population has a higher fracture incidence and greater fatality rates after a hip or vertebral fracture.[33] Daily supplementation of 400 IU (10 μg) for 2 years increased serum 25(OH)D from 10.8 to 24.8 ng/mL; moreover, it prevented the reduction of femoral neck BMD.[34] A double-blind RCT that supplemented participants ≥65 years of age with high-dose vitamin D every 4 months (100,000 IU ≈ 830 IU/day=41.5 μg/day) for 5 years resulted in an increased mean serum 25(OH)D (29.7 ng/mL) compared to the control group (21.4 ng/mL); additionally, hip and vertebral fracture risk was ameliorated without any adverse effects.[35] However, recent studies reported different observations. For instance, one study supplemented 2,000 IU/day (50 μg/day) for 3 years in participants with mean serum 25(OH)D 22.4 ng/mL with no effect on non-vertebral fractures.[36] Similarly, monthly administrations of vitamin D (12,000, 24,000, or 48,000 IU ≈ 400, 800, or 1,600 IU/day=300, 600 or 1,200 μg/day) for one year increased serum 25(OH)D from 16 ng/mL to 22.2, 25.8, or 31.6 ng/mL, respectively, but did not improve hip BMD.[37] On the other hand, high annual doses of vitamin D administration (500,000 IU ≈ 1,370 IU/day=34.3 μg/day) for 3 to 5 years significantly raised both serum 25(OH)D (to ≈ 36 ng/mL) and the risk of falls and fractures in community-dwelling elderly women (baseline 25[OH]D, 19.6 ng/mL).[33] In summary, these findings indicate that excessive vitamin D could lead to adverse outcomes on bone health and increase the risk of fractures in elderly adults. Although more scientific data is needed, the target serum 25(OH)D for relatively healthy elderly adults (≥70 years of age) may be 20 ng/mL; and vitamin D status above 30 ng/mL may not be required for this group.
3. Older adults receiving osteoporosis medications
While the influences of vitamin D status on the efficacy of osteoporosis medication may differ according to the types of medications, studies on the required vitamin D status for osteoporosis patients receiving treatments have rarely been performed. Regarding antiresorptives such as bisphosphonates, denosumab, and raloxifene, observational studies indicate that baseline serum 25(OH)D >20 ng/mL is associated with higher BMD and lower fracture risk in patients receiving bisphosphonate or raloxifene treatment.[38,39] In an RCT of participants with baseline serum 25(OH)D ≥20 ng/mL, alendronate was more effective compared to placebo.[40] On the other hand, baseline serum 25(OH)D did not affect the efficacy of risedronate, denosumab, or raloxifene on BMD or fracture risk when provided with calcium and vitamin D supplements.[41–44] In patients with 25(OH)D <30 ng/mL of whom the loss of BMD was observed prior to vitamin D treatment despite the use of bisphosphonates, increasing vitamin D status resulted in increases in BMD at the lumbar spine and the femoral neck.[45] To our knowledge, only a few efficacy trials of bazedoxifene report vitamin D status. In a small study, bazedoxifene was effective in osteoporotic women with a mean baseline 25(OH)D of 48.3 ng/mL.[46] In the aspect of adverse effects following the use of medications, serum 25(OH)D <30 ng/mL is associated with a higher risk of acute-phase response (e.g., flu-like symptoms, fever, fatigue, malaise, and myalgia) after the first dose of intravenous nitrogen-containing bisphosphonates, compared to those with higher vitamin D status.[47] Therefore, based on the currently available research, serum 25(OH)D at 30 ng/mL is safe for patients receiving antiresorptives, although only a few studies are available and dose-dependent trials are lacking.
Studies on optimal vitamin D status for patients taking anabolic agents are few, if any, especially on recently approved medications. Most studies investigating the efficacy of these medications additionally provided calcium with vitamin D supplements,[44,48] concealing the independent effect of vitamin D, whereas many did not report serum 25(OH)D during the trials.[49–51] Clinical trials found abaloparatide effective in patients with serum 25(OH)D >15 ng/mL at enrollment and supplemented with 400 to 800 IU/day (10–20 μg/day) of vitamin D.[49,52] Similarly, a trial on romosozumab was effective in women with serum 25(OH)D ≥20 ng/mL and low bone mass or osteoporosis.[40] Romosozumab was also successful in increasing BMD in patients with osteoporosis receiving calcium and vitamin D supplements, 600 to 800 IU/day (15–20 μg/day), of which those with serum 25(OH)D ≤40 ng/mL at baseline were boosted with 50,000 to 60,000 IU (1,250–1,500 μg) of vitamin D in addition to the supplements.[50,51] However, the vitamin D status at which the drug intervention was effective was not reported in most cases. Therefore, further research regarding the effect of vitamin D status on the efficacy of osteoporosis treatment is required, especially for anabolic agents.
On the other hand, more caution may be required for teriparatide, another anabolic agent. Teriparatide is effective in individuals with serum 25(OH)D >20 ng/mL [40] or in those receiving 800 IU/day (20 μg/day) of vitamin D,[53] while reported to increase 1,25(OH)2D.[54] However, the use of teriparatide in patients with high 25(OH)D may increase the risk of hypercalcemia.[55] Therefore, although more information is needed, vitamin D supplementation in patients prescribed teriparatide requires caution if serum 25(OH)D >20 ng/mL, with regular follow-up of serum calcium.
In conclusion, based on the currently available scientific evidence, the target serum 25(OH)D levels of the patients on anabolic agents (such as teriparatide, abaloparatide, and romosozumab) may be >20 ng/mL, whereas 30 ng/mL may be more beneficial for patients treated with antiresorptives (such as bisphosphonates, denosumab, and raloxifene). Additional scientific evidence is required to establish the target vitamin D status for older adults receiving medical treatment for osteoporosis. The type of medication must be considered when the optimal vitamin D status is established.
OBTAINING ADEQUATE VITAMIN D STATUS THROUGH THE INTAKE
Although South Korea is located between 33 and 43°N, an adequate latitude for cutaneous synthesis of vitamin D, the vitamin D status of Koreans is relatively low.[20,56] Theoretically, 20 to 40 min of sun exposure to the face and hands are suggested to obtain 400 IU (10 μg) of vitamin D for Koreans who live in Korea,[57] but clinical studies in Japan show that this is recommended time may vary greatly according to season, time of day, and latitude.[58]
Koreans enjoy indoor activities and actively promote sun-protective behaviors, including the use of sunscreen, sun visors, and ultraviolet (UV)-protective clothing, when they enjoy outdoor activities. In addition, obtaining sufficient vitamin D through UV exposure is likely to be more difficult in older adults since the efficiency of subcutaneous synthesis of vitamin D decreases with age.[59] Therefore, a vitamin D-rich diet and supplements may be an easier approach for Koreans to achieve adequate vitamin D status. As the dose-dependent relationship between vitamin D intake and serum 25(OH)D has not been established in Koreans, the Adequate Intake of vitamin D for Koreans is established based on the data from Whites assuming minimum UV exposure.[60] Despite the difference in body composition where Asians have higher adiposity at a given body mass index,[61] the smaller body frame of Koreans compared to North Americans was considered for the recommendations. For younger Korean adults, 400 IU/day (10 μg/day) of vitamin D is required, whereas 600 IU/day (15 μg/day) of vitamin D is recommended for older Koreans (≥65 years) in the healthy population to achieve 20 ng/mL of serum 25(OH)D.[60] The intake to reach 30 ng/mL, especially for those receiving treatment for osteoporosis and fracture prevention, is estimated to be 800 to 1,000 IU/day (20–25 μg/day) [60].
1. Dietary sources of vitamin D
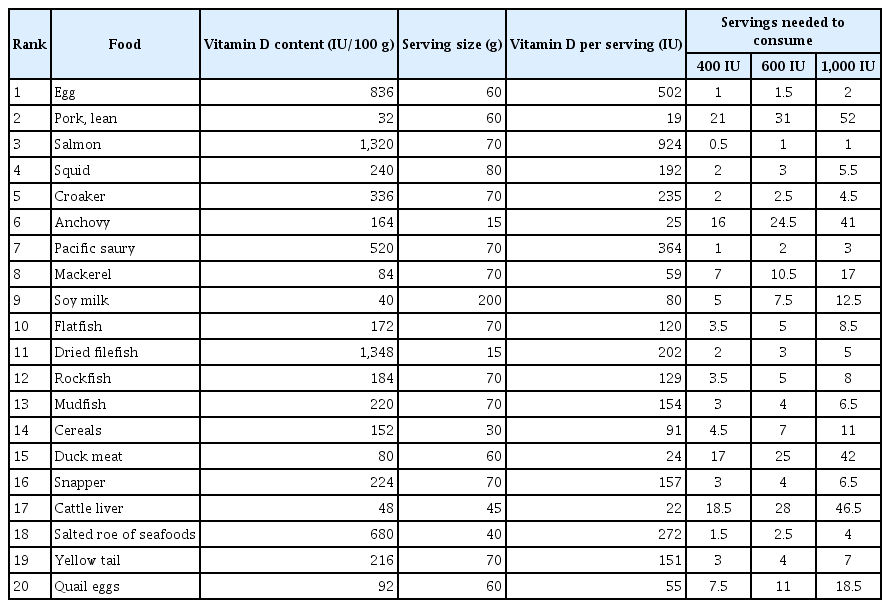
The naturally available vitamin D forms are ergocalciferol (vitamin D2), which is present in plants and fungi, and cholecalciferol (vitamin D3), which is commonly found in animal sources; and the physiological roles of the 2 forms are similar (Fig. 1).[8,62] In Korean diets, the most vitamin D-rich foods are Ayu intestine and filefish; however, these foods are not frequently consumed (Table 1). The major dietary sources of vitamin D in Koreans are eggs, lean pork, and salmon (Table 2).[60] One egg (60 g) and one serving of salmon (70 g) contain 500 IU (12.5 μg) and 924 IU (23.1 μg) of vitamin D, respectively. As seafood is popular among Koreans, the consumption of pacific saury, carp, croaker, squid, and mackerel may help to increase vitamin D intake. Despite the low vitamin D content of pork, pork is the second major source of dietary vitamin D in the Korean diet due to its frequent consumption. In general, high vitamin D-containing foods are not frequently consumed in daily diet [8,15]; therefore, many countries, including the US, Canada, and European countries mandatorily or voluntarily fortify vitamin D in the foods, such as dairy and breakfast cereals.[63,64] Vitamin D-fortified foods are affordable and simple means to effectively increase serum 25(OH)D and BMD.[65] However, vitamin D-fortified foods are not as frequently produced and consumed in Korea as in Western countries. Frequent consumption of vitamin D-rich foods and the development of various vitamin D-fortified foods will be an effective approach to increasing vitamin D status of Koreans.
2. Dietary vitamin D supplements
Dietary vitamin D is mainly composed of cholecalciferol (vitamin D3) and can be an effective means to maintain optimal vitamin D status for specific populations, such as older adults.[15,66] Indeed, in the United States, dietary vitamin D supplements account for approximately 15% of dietary vitamin D.[63,64]
Compared to other age groups, older adults (≥65 years) have a greater requirement for vitamin D, because aging decreases cutaneous vitamin D synthesis [59] and raises the resistance of 1,25(OH)2D’s actions in the intestine as well as fracture risk.[67] Furthermore, older people may also have difficulty modifying their diet habits,[68] even though they are recommended to change their diet with vitamin D-rich foods to maintain bone health. Therefore, meeting an individual’s vitamin D needs through diet alone may be challenging for older adults; thus, dietary vitamin D supplements may be an effective way to achieve adequate vitamin D status for this population. Few, side effects of dietary vitamin D supplements have been reported at reasonable doses. The most prevalent side effect of high vitamin D is hypercalcemia but it is generally observed when serum 25(OH)D is higher than 50 ng/mL.[12,69] Thus, daily supplementation of vitamin D is more frequently recommended rather than the intermittent consumption of high-dose vitamin D supplements.[70]
Individuals with diseases related to the gastrointestinal tract, liver/gall bladder, or kidney are another population that might need dietary vitamin D supplements as they may be required to restrict vitamin D-rich foods or have difficulty in the absorption of fat-soluble vitamins, including vitamin D.[71,72] For instance, anorexia owing to cancer and other diseases may decrease overall food intake, resulting in a deficient intake of overall nutrients, including vitamin D. Thus, dietary vitamin D supplements may be essential for these subjects. Additionally, patients with hepatic or renal diseases may be unable to metabolize vitamin D, increasing the need for dietary vitamin D supplements to maintain optimal 25(OH)D and/or 1,25(OH)2D levels as supplemental 25(OH)D may result in the rapid elevation and/or stabilization of serum 25(OH)D.[73] Medical professionals, including clinical physicians, must closely monitor the vitamin D status and adverse events in the patients to determine the appropriate dose of vitamin D to obtain optimal vitamin D status.
CONCLUSIONS AND REMAINING QUESTIONS
The current position statement regarding vitamin D in healthy older adults (<70 years of age) and elderly adults (≥70 years of age) having a high fracture risk or taking medications for osteoporosis updates the previous 2015 statement [21] and includes dietary recommendations for vitamin D intake to maintain skeletal health.
(1) Healthy adults should aim for serum 25(OH)D at least 20 ng/mL. However, 25(OH)D over 50 ng/mL may be detrimental to bone health and increase the risk of adverse events in older adults.
(2) Adults taking osteoporosis medications should maintain serum 25(OH)D ≥20 ng/mL. Serum 25(OH)D ≥30 ng/mL may be more effective for adults taking antiresorptive.
(3) In healthy adults, 400 IU/day (10 μg/day) is required for younger adults, while older adults (≥65 years) are recommended to consume 600 IU/day (10 μg/day) of vitamin D to reach 20 ng/mL of serum 25(OH)D. Daily intake of 800 to 1,000 IU (20–25 μg) of vitamin D is recommended for patients who aim to reach 25(OH)D at 30 ng/mL.
(4) Consumption of eggs, salmon, pork, and vitamin D supplements is a practical and safe method to reach adequate vitamin D status. In addition, the production of diverse vitamin D-fortified foods could broaden the options of dietary sources of vitamin D for Koreans.
The following are the remaining questions that require further research:
(1) What is the dose-response relationship between vitamin D intake and 25(OH)D, BMD, and fracture risk in Koreans? Few research has been performed on Koreans, most of which are observational. More intervention studies are required to assess the effect of vitamin D on bone health in Koreans.
(2) What are the requirements of vitamin D for those with secondary osteoporosis (osteoporosis due to gastrointestinal disease, liver disease, renal disease, endocrine disorders, cancer, immobilization, etc)?
(3) What dietary patterns concerning vitamin D status are beneficial to skeletal health and applicable to Koreans?
(4) How does vitamin D intake at different life stages other than in older adulthood (i.e., in utero, infancy, childhood, adolescence, pregnancy, and lactation) affect bone health, the prevalence of osteoporosis, and fracture risk?
Notes
Conflict of interest
No potential conflict of interest relevant to this article was reported.