|
 |
| jbm > Volume 29(2); 2022 > Article |
|
Abstract
Background
Methods
Results
Conclusions
DECLARATIONS
Funding
This research was supported by a grant from the Korean Health Technology R & D Project through the Korean Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI19C0481, HC20C0157). And, this work was supported by Biomedical Research Institute Fund (GNUHBRIF-2018-0008) from the Gyeongsang National University Hospital.
Fig. 1
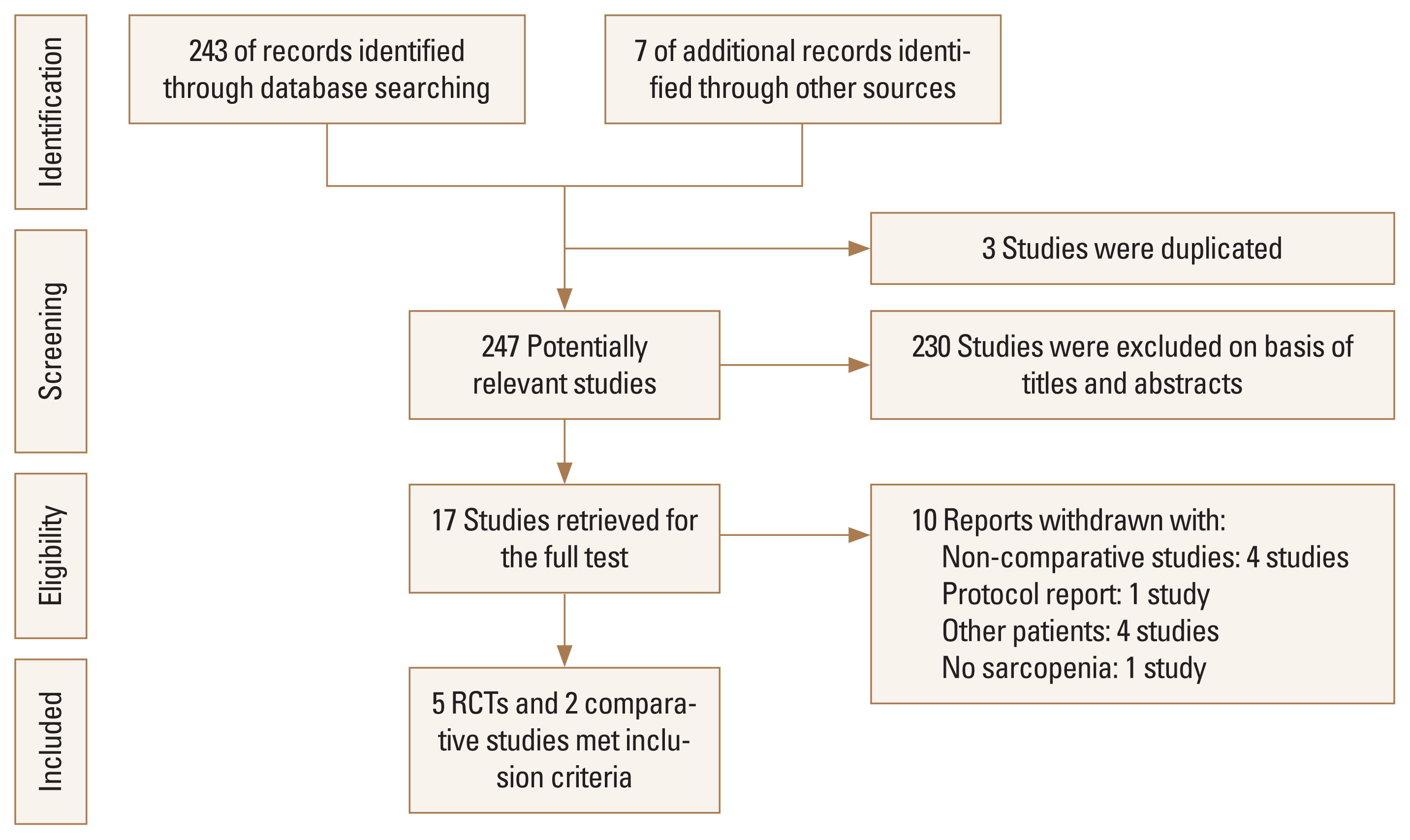
Table 1
| References | Year | Study design | Inclusion criteria | Diagnosis criteria of sarcopenia | No. of hip fractures | Type of intervention | Intervention | ||
|---|---|---|---|---|---|---|---|---|---|
| Controls | Treatment group | ||||||||
| Oh et al. [3] | 2020 | Prospective RCT | Age 65-90, sarcopenia, after hip Fx. surgery | AWG | 38 | Rehabilitation | Conventional rehabilitation (30 min from physical therapist on each of the 10 consecutive working days. Passive hip and knee mobilization, strengthening of the hip abductor and extensor muscles, transfer, and gait training on the floor and stairs during every session) | AGT combined with conventional rehabilitation in the first week (on days 1-5), AGT was applied with 50-60% of body weight administered at a rate of 1.5 mph during 20 min based on weekday. In the following weeks (on days 6-10), patients received AGT for 20 min, with 70-80% of body weight administered at a rate of 1.5-1.8 mph | |
| de Sire et al. [4] | 2020 | Prospective RCT | Age ≥65, 3 months after THA for hip Fx | EWG | 20 | Nutrition | Specific physical exercise rehabilitative program, consisting of 5 sessions of 40 min each (15 min of walking training, 10 min of upper and lower limb strengthening and stretching, and 10 min of balance exercises) per week for 2 weeks under the supervision of an experienced physical therapist, and, subsequently, a home-based exercise protocol (aerobic, flexibility, resistance, and neuromotor) without physical therapist supervision | Patients received a 2-month amino acid supplementation (Aminotrofic®, Errekappa Euroterapici Spa, Milan, Italy), 2 sachets of 4 g daily (1,250 mg of l-leucine, 650 mg of l-lysine; 625 mg of l-isoleucine, 625 mg of l-valine, 350 mg of l-threonine, 150 mg of l-cystine, 150 mg of l-histidine, 100 mg of l-phenylalanine, 50 mg of l-methionine, 30 mg of l-tyrosine, 20 mg of l-tryptophan; 0.15 mg of vitamin B6, and 0.15 mg of vitamin B1) with specific physical exercise rehabilitative program | |
| Zhang et al. [2] | 2020 | Retrospective comparative study | Age >60, after intertrochanteric Fx. surgery, sarcopenia | AWG | 141 | Medication | Patients received the erythropoietin intravenous injection (10,000 IU) once per day on the day of surgery and then continuously for 10 days. Recombinant human erythropoietin injection (CHO cell, Shenyang Sansheng Pharmaceutical, Shenyang, China) | ||
| Lim et al. [17] | 2019 | Prospective observational comparative study | Age >65, femoral neck Fx | AWG | 80 | Rehabilitation | The fragility FIRM program during 2 weeks hospital stay after surgery. The FIRM program consists of 10 days of physical therapy (2 sessions of 60-min per day), 4 days of OT, fall prevention education, discharge planning, and referral to community-based care during the-2 week hospital stay after surgery. During the FIRM program, physical therapy (weight-bearing exercises, strengthening exercises, gait training, aerobic exercise, and functional training) gradually progressed based on the individual’s functional level; OT for activity of daily living (transfer, sit-to-stand, bed mobility, dressing, self-care, and use of adaptive equipment) was also provided. Intensive education-based on an hip Fx. care manual was provided for patients and their families by members of the multidisciplinary rehabilitation team | ||
| Invernizzi et al. [1] | 2019 | Prospective RCT | Age >65, 3 months after hip Fx. surgery | EWG | 32 | Nutrition | Physical exercise rehabilitative program (5 sessions of 40 min/week for 2 weeks, followed by a home-based exercise protocol). The first phase of each session consisted of 15 min of walking training, the second phase consisted of 10 min of upper and lower limb strengthening and stretching, standing or at bed, and the fourth phase consisted of 10 min of balance exercises. Each session lasted 40 min and was performed with the supervision of an experienced physiotherapist. After these first 2 weeks of physical exercise rehabilitative program, all participants performed a home-based exercise. Protocol up to the end of the study period, after 2 months of intervention | Physical exercise rehabilitative program (5 sessions of 40 min/week for 2 weeks, followed by a home-based exercise protocol) and received a dietetic counseling; supplemented with 2 sachets of 4 g/day of essential amino acids (Aminotrofic®, Errekappa Euroterapici Spa). Patients in group A were treated for 2 months with an essential amino acid supplementation (Aminotrofic®) 2 sachets of 4 g per day (1,250 mg of l-leucine, 650 mg of l-lysine; 625 mg of l-isoleucine, 625 mg of l-valine, 350 mg of l-threonine, 150 mg of l-cystine, 150 mg of l-histidine, 100 mg of l-phenylalanine, 50 mg of l-methionine, 30 mg of l-tyrosine, 20 mg of l-tryptophan; 0.15 mg of vitamin B6, and 0.15 mg of vitamin B1) | |
| Malafarina et al. [8] | 2017 | Multicenter prospective RCT | Age >65, after hip Fx. surgery | EWG | 107 (drop-out 15) | Nutrition | Rehabilitation therapy comprised 2 distinct parts. The first part took place in the hospital ward (nursing staff and occupational therapist) and was based on moving patients early using technical aids (canes, crutches, or walker), and rehabilitation of activities of daily living. The second part (physical therapy) took place at the hospital gym and included exercises to strengthen the lower limbs, balance exercises and walking re-training in individual or group 50 min sessions, once a day 5 days a week (Monday to Friday) | Rehabilitation therapy with standard diet plus oral nutritional supplementation in the form of 2 bottles a day of HMB. The nutritional characteristics of the standard diet are: 1,500 Kcal, 23.3% protein (87.4 g/day), 35.5% fat (59.3 g/day) and 41.2% carbohydrates (154.8 g/day). In addition, patients in the IG received 2 bottles a day (1 in the morning and 1 in the afternoon) of prepared oral liquid nutritional supplementation (220 mL×2, total: 660 Kcal) (Ensure® Plus Advance, Abbott Laboratories, Chicago, Il, USA) with the following nutritional characteristics: 1.5 Kcal/mL, 24% protein (9.1 g/100 mL), 29% fat (5 g/100 mL) and 46% carbohydrates (16.8 g/100 mL). The supplement was enriched with CaHMB 0.7 g/100 mL, 25(OH)D 227 IU/100 mL and 227 mg/100 mL of calcium | |
| Flodin et al. [5] | 2015 | Prospective RCT | Age >60, after hip Fx. surgery | EWG | 79 | Nutrition | Received calcium 1 g and vitamin D 800 IE; specifically, cholecalciferol (Calcichew-D3®, Takeda Pharmaceutical Company Limited, Osaka, Japan) divided into 2 daily doses for 12 months | Ca, vitamin D + Risedronate (Optinate®, Warner Chilcott, Weiterstadt, Germany) 35 mg once weekly for 12 months | Ca, vitamin D + Risedronate + nutritional supplement a 200 mL package twice daily, each containing 20 g of protein and 300 Kcal (Fresubin®, Fresenius Kabi, Bad Homburg, Germany). This supplement was given for the first 6 months following hip Fx |
Table 2
| References | Intervention | No. of hip fractures | No. of sarcopenia | Age | Sex (F:M) | Height (cm) | Weight (kg) | BMI (g/m2) | Assessment methods | Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|
| Oh et al. [3] | Controls | 19 | 19 | 81.15±4.9 | 13:6 | 157.73±7.53 | 52.08±11.62 | 20.93±4.54 | Koval walking ability scores functional ambulatory category, Berg Balance Scale, Korean version of Mini-Mental State Examination, Euro Quality of Life Questionnaire Five-Dimensional Classification, Korean version of modified Barthel index, and grip strength | Both groups were improved after intervention. As additional benefits were evident among those who carried out antigravity treadmill, it may be appropriate for patients with sarcopenia after hip fracture surgery |
| Treatments | 19 | 19 | 76.94±9.43 | 13:6 | 160.08±8.25 | 55.51±11.15 | 21.58±3.23 | |||
|
|
||||||||||
| de Sire et al. [4] | Controls | 10 | 8 | 77.65±8.4 | 8:2 | Serum myostatin level, skeletal muscle mass index, obtained by whole-body tetrapolar bioelectrical impedance analysis (BIA 101 Anniversary Sport Edition, Akern Srl, Florence, Italy); appendicular muscle strength, measured by hand-grip strength test (hand-held Jamar® dynamometer); and physical performance, using the TUG | In this proof of principle study, we found a significant intragroups difference in terms of serum myostatin levels in both groups. On the other hand, we found no significant differences between groups in serum myostatin levels at the end of treatment, probably due to the correlation between physical exercise protocol and myostatin levels, independently from amino acids supplementation | |||
| Treatments | 10 | 7 | 80.33±6.72 | 9:1 | ||||||
|
|
||||||||||
| Zhang et al. [2] | Controls | 33 | 33 | 78.63±7.28 | 33:0 | Appendicular skeletal muscle mass, hand-grip strength measurement, Hemoglobin level | Erythropoietin can improve the muscle strength of female patients with sarcopenia during the perioperative period, and increase muscle mass both of women and men | |||
| Controls | 22 | 22 | 75.01±8.2 | 0:22 | ||||||
| Treatments | 44 | 44 | 79.54±6.2 | 44:0 | ||||||
| Treatments | 39 | 39 | 76.97±7.71 | 0:39 | ||||||
|
|
||||||||||
| Lim et al. [17] | Controls | 45 | 0 | 79.7±6.5 | 34:11 | 157.6±7.1 | 56.7±8.1 | 22.9±3.5 | Main outcomes for ambulatory function (Koval score, Functional Ambulatory Category) and other secondary outcomes were measured at rehabilitation admission, at discharge, at 3 months and 6 months after surgery. Other secondary outcomes were measured. The possibility of independent ambulation at 6 months after surgery were also investigated | The fragility fracture integrated rehabilitation management program was effective for promoting functional recovery in older patients with fragility hip fracture, either with or without sarcopenia |
| Treatments | 35 | 35 | 82.8±7.5 | 28:7 | 153.6±8.4 | 48.5±9.1 | 21.2±3.4 | |||
|
|
||||||||||
| Invernizzi et al. [1] | Controls | 16 | 12 | 77.65±8.4 | 14:3 | 23.15±5.33 | Hand-grip strength, TUG, and Iowa Level of Assistance Scale | A multidisciplinary rehabilitative and nutritional intervention seems to be effective on functioning in hip fracture patients, in particular sarcopenic ones | ||
| Treatments | 16 | 11 | 80.33±6.72 | 13:2 | 23.05±4.77 | |||||
|
|
||||||||||
| Malafarina et al. [8] | Controls | 43 | 84.7±6.3 | 35:9 | 160±1.0 | 63.2±14.7 | 26±5.4 | Body mass index, anthropometric parameters, Barthel index and the Functional Ambulation Categories score. Muscle mass was assessed using bioelectrical impedance analysis, which allowed us to calculate appendicular lean mass | A diet enriched in HMB improves muscle mass, prevents the onset of sarcopenia and is associated with functional improvement in elderly patients with hip fractures. Orally administered nutritional supplements can help to prevent the onset of sarcopenic obesity | |
| Treatments | 49 | 85.7±6.5 | 33:10 | 160±1.0 | 62.7±12.9 | 24.9±4.4 | ||||
|
|
||||||||||
| Flodin et al. [5] | Controls | 25 | 78±11 | 19:6 | 22.4±2.6 | Body composition as measured by dual-energy X-ray absorptiometry, HGS and HRQoL were registered at baseline, 6 and 12 months postoperatively | Protein-rich nutritional supplementation was unable to preserve fat-free mass index more effectively than vitamin D and calcium alone, or combined with bisphosphonate, in this relatively healthy group of hip fracture patients. However, trends toward positive effects on both HGS and HRQoL were observed following nutritional supplementation | |||
| Controls | 28 | 80±9 | 18:10 | 24±2.9 | ||||||
| Treatments | 26 | 81±8 | 19:7 | 22.7±3.4 | ||||||
REFERENCES
- TOOLS
-
METRICS

-
- 3 Crossref
- 0 Scopus
- 3,221 View
- 144 Download
- ORCID iDs
-
Jun-Il Yoo

https://orcid.org/0000-0002-3575-4123Yong-Chan Ha

https://orcid.org/0000-0002-6249-0581Yonghan Cha

https://orcid.org/0000-0002-7616-6694 - Related articles
-
Vitamin D Deficiency and Sarcopenia in Hip Fracture Patients2021 February;28(1)
Does Teriparatide Improve Fracture Union?: A Systematic Review2020 August;27(3)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print