Effect of Cornus Officinalis on Receptor Activator of Nuclear Factor-kappaB Ligand (RANKL)-induced Osteoclast Differentiation
Article information
Abstract
Objectives
Osteoporosis is a disease of bones that is thought to result from an imbalance between bone resorption and bone formation. Although osteoporosis itself has no symptoms, osteoporosis caused by osteoclasts leads to an increased risk of fracture. Here we examined the effects of cornus officinalis on receptor activator of nuclear factor-kappaB ligand (RANKL)-mediated osteoclast differentiation.
Methods
We evaluated the effects of cornus officinalis on RANKL-induced osteoclast differentiation from bone marrow-derived macrophages (BMMs) and performed a cytotoxicity assay, reverse transcriptase-polymerase chain reaction (RT-PCR), and Western blot analysis.
Results
Cornus officinalis significantly inhibits RANKL-mediated osteoclast differentiation in a dose-dependent manner, but without cytotoxicity against BMMs. The mRNA expression of tartrate-resistant acid phosphatase (TRAP), osteoclast-associated receptor (OSCAR), c-Fos, and nuclear factor of activated T cells cytoplasmic 1 (NFATc1) in BMMs treated with RANKL was considerably inhibited by cornus officinalis treatment. Also, cornus officinalis inhibits the protein expression of c-Fos and NFATc1. Cornus officinalis greatly inhibits RANKL-induced phosphorylation of p38 and c-JUN N-terminal kinase (JNK). Also, cornus officinalis significantly suppresses RANKL-induced degradation of I-κB.
Conclusions
Taken together, our results suggest that cornus officinalis may be a useful the treatment of osteoporosis.
INTRODUCTION
Bone mass is maintained by a homeostatic balance between the activities of osteoclasts, which resorb bone, and those of osteoblasts, which make bone. The balance between both cells is under the control of various hormones and cytokines.[1] Excessive osteoclast activity leads to bone loss in pathologic or inflammatory conditions, such as rheumatoid arthritis and postmenopausal osteoporosis.[2]
Osteoclasts originate from hematopoietic stem cell monocyte/macrophage lineage that differentiate and fuse into multinucleated bone resorbing osteoclasts in the presence of tumor necrosis factor (TNF) family, receptor activator of nuclear factor-kappaB (RANK) ligand (RANKL) and macrophage colony-stimulating factor (M-CSF).[2-4] M-CSF is a secreted cytokine from osteoblasts/stromal cells and induces colony formation of monocyte/osteoclast precursor cells. RANKL is expressed on the membrane of osteoblasts/stromal cells and binds to and activates RANK on osteoclast precursor cells.[3,5] Upon binding to its receptor RANK, RANKL initiates the recruitment of TNF-associated factor 6 (TRAF6) and immunoreceptor tyrosinebased activation motif (ITAM)-containing adaptor proteins, DAP12 and FcRγ. Then, RANKL activates the intracellular signaling pathways, including mitogen-activated protein kinase (MAPK) and nuclear factor-kappaB (NF-κB).[5,6] Ultimately, RANKL leads to osteoclast differentiation by inducing transcription factors, including c-Fos and nuclear factor of activated T cells cytoplasmic 1 (NFATc1).[7] In particular, NFATc1 rescues osteoclast differentiation in cells lacking c-Fos and can induce osteoclast differentiation in the presence of RANKL.[8]
Recently, many plant-derived natural products have been used in traditional medicine for the treatment of various diseases. We previously reported that several extracts derived from natural products, including papaya, deer antler, and drynariae rhizome, had inhibitory effects on osteoclast differentiation.[9-11] Cornus officinalis, a species of dogwood, was used as a food plant. In particular, the fruit of cornus officinalis was widely used for its chemotherapeutic benefits. Recently, cornuside, isolated from the fruit of cornus officinalis, suppressed inflammation by inhibiting TNF-alpha (α)-induced NF-κB p65 translocation to the nucleus.[12] Cornuside has also been reported to inhibit lipopolysaccharide-induced inflammation through inhibition of NF-κB activity.[13] Recently, an aqueous extract prepared from the fruit of a popular Chinese herb Cornus officinalis had strong anti-proliferative effect on estrogen receptor-positive breast cancer cells.[14]
Taken together, it is possible that an extract obtained from Cornus officinalis suppresses RANKL-induced osteoclast differentiation by inhibiting NF-κB activity. Thus, we examined the effects of Cornus officinalis on osteoclast differentiation.
MATERIALS AND METHODS
1. Reagents
An extract of cornus officinalis was from Plant Extract Bank (Yuseong, Daejeon, Korea). Human RANKL and M-CSF were from PeproTech EC Ltd. (London, UK). Anti-actin antibody and tartrate-resistant acid phosphatase (TRAP) staining kit were from Sigma-Aldrich (St. Louis, MO, USA). The 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2 tetrazolium hydroxide (XTT) assay kit was from Roche (Indianapolis, IN, USA). Antibodies against c-Fos and NFATc1 were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Antibodies against phosphor (p)-38, p38, p-c-JUN N-terminal kinase (JNK), JNK, and I-κB were from Cell Signaling Technology (Beverly, MA, USA).
2. Osteoclast differentiation
Bone marrow cells (BMCs) were freshly isolated from the long bones of 5-week-old male ICR mice as described previously.[15] Briefly, BMCs were isolated from long bones by flushing with α-minimum essential medium (α-MEM; Gibco BRL, Grand Island, NY, USA) containing antibiotics (Sigma). Red blood cells (RBC) were removed in RBC lysis buffer (Sigma). Following centrifugation, cells were suspended in α-MEM supplemented with 10% fetal bovine serum (FBS; Gibco BRL, Grand Island, NY, USA) and antibiotics, and then cultured for 3 d in the presence of M-CSF. Non-adherent cells were removed and adherent cells were used as bone marrow-derived macrophages (BMMs). BMMs were seeded at 3.5 × 104 cells/well in 48-well plates and cultured for 4 d with M-CSF and RANKL in the presence or sbaence of Cornus officinalis. Osteoclasts were stained with TRAP solution and counted as osteoclasts.
3. Cytotoxicity assay
BMMs were seeded at 1 × 104 cells/well in 48-well plates in triplicate and cultured for 3 d with M-CSF in the presence or absence of Cornus officinalis. After 3 days, 50 µL of XTT reagent was added to each well and further incubated for 6 h. The optical density at 450 nm was analyzed using an enzyme linked immunosorbent assay (ELISA) reader.
4. Western blotting
Cells were lysed in lysis buffer containing 50 mM Tris-Cl, 150 mM NaCl, 5 mM ethylenediaminetetraacetic acid (EDTA), 1% Triton X-100, 1 mM sodium fluoride, 1 mM sodium vanadate, 1% deoxycholate, and protease inhibitors. Protein concentrations were determined by using a Bio-Rad Protein assay kit. Equal amounts of proteins were boiled in SDS sample buffer, resolved by 10% SDS-polyacrylamide gel electrophoresis (PAGE), and transferred to a polyvinylidene fluoride (PVDF) membrane (Amersham Biosciences, Piscataway, NJ, USA). The membrane was blocked and probed with primary antibodies, as indicated, washed extensively, and then incubated with secondary antibody conjugated to horseradish peroxidase. Protein-antibody complexes were detected with enhanced chemiluminescence (ECL; Amershan Biosciences, Buckinghamshire, UK).
5. RNA isolation and reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted from cultured cells using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. One µg of RNA wasreverse-transcribed using oligo dT primers, deoxynucleotide triphosphate (dNTP), buffer, dithiothreitol, RNase inhibitor, and Superscript II RT (Invitrogen, Carlsbad, CA, USA). cDNA was amplified using the following primer sets: TRAP sense, 5'-ACTTCCCCAGCCCTTACTAC-3'; TRAP antisense, 5'-TCAGCACATAGCCCACACCG-3'; c-Fos sense, 5'-CTGGTGCAGCCCACTCTGGTC-3'; c-Fos antisense, 5'-CTTTCAGCAGATTGGCAATCTC-3'; NFATc1 sense, 5'-CAACGCCCTGACCACCGATAG-3'; NFATc1 antisense, 5'-GGCTGCCTTCCGTCTCATAGT-3'; osteoclast-associated receptor (OSCAR) sense, 5'-GAACACCAGAGGCTATGACTGTTC-3'; OSCAR antisense, 5'-CCGTGGAGCTGAGGAAAAGGTTG-3'; GAPDH sense, 5'-ACCACAGTCCATGCCATCAC-3'; GAPDH antisense, 5'-TCCACCACCCTGTTGCTGTA-3'. PCR products were electrophoresed by using 1% agarose gels and visualized by staining with ethidium bromide. Densitometric analysis was performed on the relative intensity of each band using the Image Pro-plus program, version 4.0 (Media Cybernetics, Carlsbad, CA, USA).
6. Statistics
Statistical significance was analyzed by a Student's t test, and significant results (P < 0.1) are marked by asterisks.
RESULTS
1. Effects of cornus officinalis on osteoclast differentiation
To examine the effect of cornus officinalis on osteoclast differentiation, BMMs were cultured with M-CSF and RANKL in the presence or absence of cornus officinalis. Osteoclasts were formed from BMM in the presence of M-CSF, RANKL, and dissolved in dimethyl sulfoxide (DMSO) as vehicle. However, cornus officinalis greatly inhibits the differentiation of BMMs, as evaluated by the number of TRAP-positive osteoclasts (Fig. 1A, 1B). To exclude the possibility that cornus officinalis can cause cytotoxicity on BMMs during osteoclast differentiation, the cytotoxicity assay was performed. Cornus officinalis can no cytotoxic effects at the same doses which considerably inhibited osteoclast differentiation (Fig. 1C).
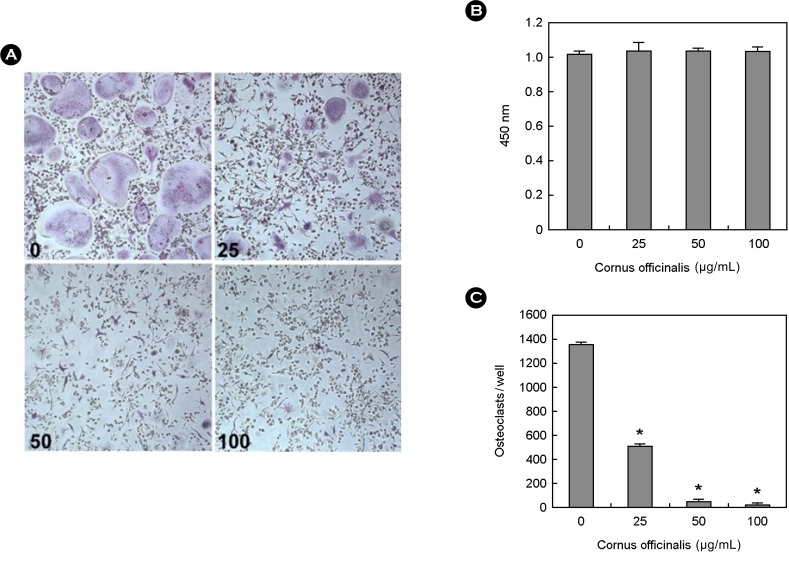
Cornus officinalis inhibits osteoclast differentiation. (A) Bone marrow macrophages (BMMs) were cultured for 4 d with macrophage colony-stimulating factor (M-CSF; 30 ng/mL) and receptor activator of nuclear factor-kappaB ligand (RANKL; 100 ng/mL) in the presence or absence of Cornus officinalis. After 4 d, cells were fixed in 3.7% formalin, permeabilized in 0.1% Triton x-100, and stained for tartrate-resistant acid phosphatase (TRAP). (B) TRAP-positive cells were counted as osteoclasts. (C) BMMs were cultured 3 d with M-CSF (30 ng/mL) in the presence or absence of Cornus officinalis. After 3 d, 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2 tetrazolium hydroxide (XTT) solution was added to each well and then incubated for 4-6 h. The plate was measured at 450 nm using a microplate reader. Experiments were performed in triplicate and similar results were obtained in two independent experiments.
2. Effects of cornus officinalis in osteoclast-specific gene expression
Next, we examined the inhibitory effect of cornus officinalis on osteoclast differentiation by analyzing osteoclast-specific gene expression and transcription factors. BMMs stimulated with M-CSF and RANKL showed high expression levels of TRAP and OSCAR mRNA after 48 h. However, cornus officinalis inhibits the expression of TRAP and OSCAR mRNA in BMMs treated with M-CSF and RANKL. Also, RT-PCR results revealed that c-Fos and NFATc1 mRNA levels were increased by M-CSF and RANKL, but both c-Fos and NFATc1 expression was greatly inhibited by cornus officinalis (Fig. 2). These results suggest that cornus officinalis may inhibit osteoclast differentiation through the inhibition of RANKL-induced c-Fos and NFATc1 expression.

Cornus officinalis inhibits osteoclast-specific gene expression. bone marrow-derived macrophages (BMMs) were treated with or without Cornus officinalis (100 µg/mL) and further stimulated with receptor activator of nuclear factor-kappaB ligand (RANKL; 100 n/mL) for the indicated time. cDNA was synthesized by using total RNA and reverse transcriptase-polymerase chain reaction (RT-PCR) was performed using cDNA. PCR products were separated by electrophoresis in an agarose gel and visualized with ethidium bromide staining. Similar results were obtained in three independent experiments. DMSO, dimethyl sulfoxide; TRAP, tartrate-resistant acid phosphatase; OSCAR, osteoclast-associated receptor; NFATc1, nuclear factor of activated T cells cytoplasmic 1, GAPDH, glyceraldehyde-3-phosphate dehydrogenase
3. Effects of cornus officinalis on the protein expression of c-Fos and NFATc1 induced by RANKL
Osteoclast differentiation is tightly regulated by the induction of various transcription factors. In particular, c-Fos and NFATc1 play an essential role in osteoclast differentiation.[2,7] To further confirm the above results, we examined the effects of cornus officinalis on c-Fos and NFATc1 protein expression. BMMs were stimulated with M-CSF and RANKL in the presence or absence of cornus officinalis. As shown in Figure 3, cornus officinalis suppresses the protein expression of c-Fos and NFATc1 in BMMs treated with M-CSF and RANKL. These results suggest that the inhibitory effects of cornus officinalis involved the inhibition of c-Fos and NFATc1 induced by RANKL.

Cornus officinalis inhibits the expression of c-Fos and nuclear factor of activated T cells cytoplasmic 1 (NFATc1). Bone marrow-derived macrophages (BMMs) were pretreated with or without Cornus officinalis (100 µg/mL) and further stimulated with receptor activator of nuclear factor-kappaB ligand (RANKL; 100 n/mL) for the indicated time. The cells were lysed and proteins were revolved by 10% SDS-polyacrylamide gel electrophoresis (PAGE) and subjected to Western blot analysis. Similar results were obtained in three independent experiments. DMSO, dimethyl sulfoxide
4. Effects of cornus officinalis on RANKL-induced early signaling
It is well known that RANKL activates multiple intracellular signaling cascades that lead to osteoclast differentiation.[7] To examine the molecular mechanism of the inhibitory effects of cornus officinalis on osteoclast differentiation, we examined the effects of cornus officinalis on the early signaling pathways in BMMs treated with RANKL. RANKL-induced phosphorylation of p38 and JNK was increased at 5 min after RANKL treatment, but both p38 and JNK phosphorylation was inhibited by cornus officinalis treatment. Also, RANKL induces degradation of I-κB within 5 min, but cornus officinalis suppresses I-κB degradation (Fig. 4). These results suggest that the inhibition of early signaling pathways is, at least, involved in the cornus officinalis-induced inhibition of transcription factors and osteoclast differentiation.
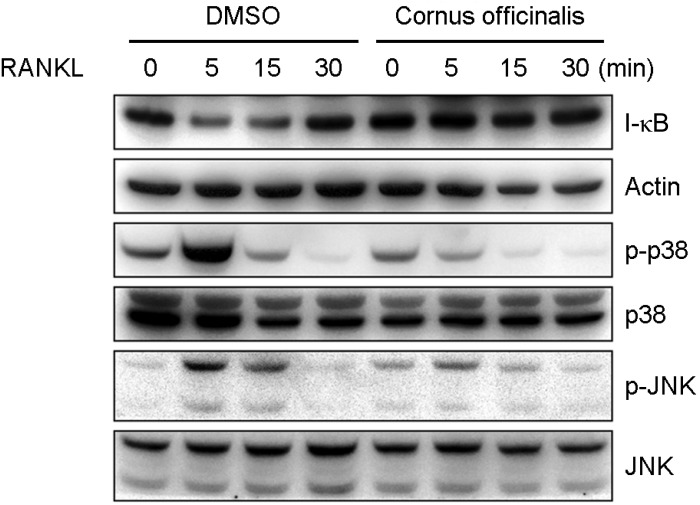
Cornus officinalis inhibits receptor activator of nuclear factor-kappaB ligand (RANKL) signaling pathways. Bone marrow-derived macrophages (BMMs) were pretreated with or without Cornus officinalis (100 µg/mL) and further stimulated with RANKL (100 n/mL) for the indicated time. The cells were lysed and proteins were revolved by 10% SDS-polyacrylamide gel electrophoresis (PAGE) and subjected to Western blot analysis. Similar results were obtained in three independent experiments. DMSO, dimethyl sulfoxide; JNK, c-JUN N-terminal kinase
DISCUSSION
In human, the function of bones is to mechanically support the body, protect the soft vital organs such as brain and heart, serve as levers for muscle action, store and maintain mineral, and support hematopoiesis. Thus, osteoporosis is one of the major health problems in our world and is especially troublesome for postmenopausal females who suffer from estrogen deficiency.[1] It is well known that an imbalance between bone-resorbing osteoclasts and bone-forming osteoblasts is the main pathogenesis of bone diseases including osteoporosis.[2-4] Osteoporosis, most common metabolic bone disorder, is one of the major age-related diseases and responsible for increasing bone fracture risk.[16]
Upon binding to RANKL, RANK activates various transcription factors including c-Fos and NFATc1 which thereby induces osteoclast-specific gene expression including TRAP and OSCAR.[5-7] In this study, Cornus officinalis inhibits RANKL-mediated osteoclast differentiation in a dose-dependent manner without cytotoxicity against BMMs (Fig. 1). Also, we found that Cornus officinalis suppresses the expression of TRAP, OSCAR, c-Fos, and NFATc1 in BMMs treated with RANKL and M-CSF. These results suggest that Cornus officinalis inhibits osteoclast differentiation through inhibition of c-Fos and NFATc1 induced by RANKL.
Although the mechanism of RANKL-induced c-Fos expression in BMMs remains unclear, they are likely to be related to NF-κB and MAPK, such as p38 and JNK.[17-19] Both c-Fos and NFATc1 are key transcription factors on osteoclast differentiation.[18] c-Fos is an upstream component relevant to NFATc1 during RANKL-induced osteoclast differentiation.[7] It has recently been reported that RANKL can induce osteoclast differentiation from NF-κB p50/p52 double knock-out (dKO) osteoclast precursor cells when c-Fos is overexpressed.[20] Also, several reports have indicated that p38 MAPK regulates RANKL-mediated c-Fos expression in osteoclast precursors.[18,21] An extract of Cornus officinalis, cornuside, suppresses inflammation by inhibiting NF-κB activity.[12,13] This raises the possibility that Cornus officinalis may regulate osteoclast differentiation through inhibition of c-Fos expression via inhibition of NF-κB activity. NF-κB is usually accumulated in the cytoplasm through association with its inhibitory protein I-κB. Following cellular stimulation, I-κB is phosphorylated, ubiquitinated, and degraded by the proteasome, which leads to the translocation of NF-κB into the nucleus, where it acts as a transcription factor.[22] In this study, I-κB was shown to undergo degradation within 5 min after treatment with RANKL. However, RANKL-induced I-κB degradation was greatly inhibited in the presence of cornus officinalis. Also, cornus officinalis significantly inhibited the phosphorylation of p38 and JNK induced by RANKL (Fig. 4). These results demonstrate that Cornus officinalis may inhibit osteoclast differentiation that may be involved in the inhibition of the signaling cascades NF-κB/c-Fos/NFATc1.
CONCLUSION
Single compounds obtained from Cornus officinalis is known to inhibit inflammation by regulating NF-κB. In this study, we found that an extract derived from cornus officinalis inhibited RANKL-induced osteoclast differentiation without cytotoxicity. The expression of c-Fos and NFATc1, the master transcription factors of osteoclast differentiation, was inhibited by cornus officinalis treatment. Cornus officinalis inhibited p38 and JNK phosphorylation and I-κB degradation in BMMs treated with RANKL.
Notes
This paper was supported by Wonkwang University in 2010.