|
 |
| jbm > Volume 27(1); 2020 > Article |
|
Abstract
A systematic search was conducted and relevant studies that evaluated the influence of osteoporosis medications (bisphosphonates [BPs], denosumab, selective estrogen receptor modulators [SERMs], recombinant human parathyroid hormone teriparatide [TPTD], and strontium ranelate [SrR]) on wrist, hip, and spine fracture healing, were selected. BPs administration did not influence fracture healing and clinical outcomes after distal radius fracture (DRF). Similar results were observed in hip fracture, but evidence is lacking for spine fracture. Denosumab did not delay the non-vertebral fractures healing in one well-designed study. No studies evaluated the effect of SERMs on fracture healing in humans. One study reported shorter fracture healing times in TPTD treated DRF patients, which was not clinically meaningful. In hip fracture, recent studies reported better pain and functional outcomes in TPTD treated patients. However, in spine fracture, recent studies found no significant differences in fracture stability between TPTD treated patients and controls. Evidence is lacking for SrR, but it did not influence wrist fracture healing in one study. In comparisons between TPTD and BPs, fracture healing and physical scores were not significantly different in hip fracture by 1 study. In spine fracture, controversy exists for the role of each medication to the fracture stability, but several studies reported that fracture site pain was better in TPTD treated patients than BPs treated patients. Considering no clinical data of negative fracture healing of the antiresorptive medication and the danger of subsequent fracture after initial osteoporotic fracture, there is no evidence to delay initiation of osteoporosis medications after fracture.
The purpose of osteoporosis evaluations and treatments is to prevent a primary osteoporotic fractures or subsequent osteoporotic fractures after an initial fracture. Despite the fact that osteoporosis is easy to diagnose and there have been various osteoporosis medications available to prescribe, evaluations and treatments were not adequately performed.[1] This phenomenon is named to ‘care gap’ and patients who experienced a recent osteoporotic fracture represent an appropriate target group to reduce this care gap.[2,3] To manage those patients properly, it is essential to understand how osteoporosis medications influence fracture healing. This knowledge is also important for patients with osteoporotic fracture who also have a history of taking osteoporosis medications or who are currently taking osteoporosis medications. We aimed to review how osteoporosis medications influence on osteoporotic fracture healing.
In this study, most popular osteoporosis medications in market: bisphosphonates (BPs), denosumab, and selective estrogen receptor modulators (SERMs) in antiresorptive medications and parathyroid hormone (PTH) analogs and strontium ranelate (SrR) in anabolic agents were reviewed. In accordance with the type of medications, details of medication administration such as timing, duration, and quantity were evaluated. For the fracture type, influences on wrist, hip, and spine fractures, which are the representative osteoporotic fractures, were evaluated.
We performed this systematic review based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A systematic search was conducted across the Cochrane Library, PubMed, and EMBASE databases (Table 1) and relevant articles were selected in September 2019 for articles published in English from 2000 onward. In order to avoid missing any relevant studies, the use of limits was restricted, and further selection was conducted manually. The references of identified articles and reviews were also checked for relevance.
BPs, widely used in the treatment of osteoporosis,[4] have powerful inhibitory effects on bone remodeling by inhibiting osteoclast activity.[5] They attach to hydroxyapatite binding sites on bony surfaces, especially surfaces undergoing active bone resorption. Therefore, there are concerns that BPs may interfere with fracture healing or adversely affect functional recovery after fracture.[6] On the contrary to the concern, several animal studies found that BPs preferentially deposit at the acute fracture site and increased callus formation for mechanical functioning, but inhibited bone remodeling by modulation of callus morphology.[7] For the timing of administration, 1 to 2 weeks delayed administration of bolus-dosed BPs yielded the callus with the greater size and strength and more superior mechanical properties compared to weekly administration.[8,9] These results suggest that bolus-dosed BPs may effectively target the fracture site after the initial anabolic fracture response and generate a larger, stronger callus.[7]
The influence of BPs to the healing of wrist fracture has been studied from early 2000s and among osteoporosis medications, BPs are most widely evaluated until now. Van der Poest Clement et al. [10] first published the results of a prospective randomized controlled trial (RCT) which compared between alendronate and a placebo in patients with distal forearm fracture and reported no significant difference between the 2 groups in fracture healing rate, but the bone mass increase was observed in alendronate treated patients. Two studies from the same group compared current BP users with BP naive patients regarding conservatively treated distal radius fracture (DRF) patients. These studies found no clinically significant differences in fracture healing time and no differences in clinical or functional outcomes between the 2 groups.[11,12] Two other studies evaluated the influence of alendronate administration timing on DRF healing after open reduction internal fixation and concluded that early administration did not impair the radiographic or clinical outcomes.[13,14] Recently, a large multicenter randomized placebo-controlled trial (RPCT) was performed in the UK to evaluate the effect of weekly alendronate on DRF healing. The investigators started alendronate 70 mg within 14 days after fracture occurrence that was treated either surgically or conservatively. They concluded that early administration of alendronate does not adversely affect fracture union or clinical outcomes.[15]
In patients with hip fracture, BP treatment showed decrease in bone turnover markers and anti-resorptive effect. Altintaş et al. [16] reported that urine N-telopeptide level significantly decreased at the end of 3 months of treatment with risedronate. In addition, Cecilia et al. [17] reported that alendronate treatment increased proximal femoral bone mineral densities (BMDs) and decreased bone turnover markers. In a large multicenter RPCT, intravenous zoledronate administered within 90 days after hip fracture was not associated with a significant delay on fracture healing.[18] In another study, the early administration of risedronate did not influence on the functional outcomes and complication in patients with intertrochanteric fracture who were treated with surgery, like in surgically treated patients with DRF.[19] However, recently Lim et al. [20] reported that history of BPs administration was associated with an increased risk of delayed union at 3 months in patients with surgically treated intertrochanteric fractures.
The influence of BPs on osteoporotic vertebral fracture healing has not been evaluated well. In one prospective study, current usage of BPs did not significantly affect the clinical outcomes, but patients treated with BPs developed intervertebral clefts which could be an indicative of impaired vertebral fracture healing (Tables 2 and 3).[21]
Denosumab is a potent inhibitor of osteoclast mediated bone resorption and is expected to have similar properties to BPs with respect to fracture healing.[22] Like BPs, denosumab does not appear to impair fracture healing in animal studies.[7] In animals treated with denosumab, callus volume increased at the fracture site and remodeling was delayed. In addition, denosumab has been found to increase torsional rigidity of the fracture site in experiments with mouse femurs.[23]
There is little published clinical data regarding fracture healing in denosumab-treated patients apart from the FREEDOM trial. In this large, multi-institution, double-blind placebo-controlled study, 7,808 postmenopausal women were randomly assigned to receive either denosumab or a placebo control and 667 patients had a total of 851 non-vertebral fractures during study period. Neither delayed healing nor nonunion was observed in any subject who had received denosumab within 6 weeks preceding or following the fracture. The complication rates associated with the fracture or intervention were not significantly different between the denosumab and placebo groups. The investigators concluded that denosumab did not delay fracture healing nor did it contribute to other complications, even when administered around the time of the fracture.[24]
SERMs provide the beneficial effects of estrogen on skeletal tissue without negative effects on other organs.[25] In an in vitro study, raloxifene, which is the main SERM used in treating osteoporosis, decreased the rate of bone remodeling and attenuated osteoclast activity but maintained osteoblast activity.[26] In a study using ovariectomized rats, both estrogen or raloxifene suppressed callus remodeling mildly and did not impede progression of fracture repair.[27] In the same mouse model, both drugs yielded calluses with larger chondrocyte areas, greater mineralization, increased trabecular and neocortical thickness, and decreased time to fracture healing compared to controls.[28,29] Those phenomena occurred both in metaphyseal and diaphyseal bones. However, there are no studies evaluating the influence of estrogen or raloxifene on fracture healing in humans.
Intermittent injection of the recombinant human PTH (teriparatide [TPTD]) is a potent anabolic agent to increase BMD in osteoporotic patients. PTH increases osteoblast function and lifespan and results in increased bone formation on all bone surfaces including endosteal bone, periosteal bone, and trabeculae.[30,31] It also increases trabecular connectivity and cortical bone thickness, which enhances biomechanical properties.[7] In an animal study, TPTD has even been shown to enhance chondrocyte recruitment and differentiation, which are essential processes in early endochondral ossification.[32] Consequently, TPTD influence both cartilaginous and mineralized callus formation in the fracture healing process.[33] For the timing of administration, optimal fracture healing was observed with early treatment within one week after fracture occurrence.[34,35]
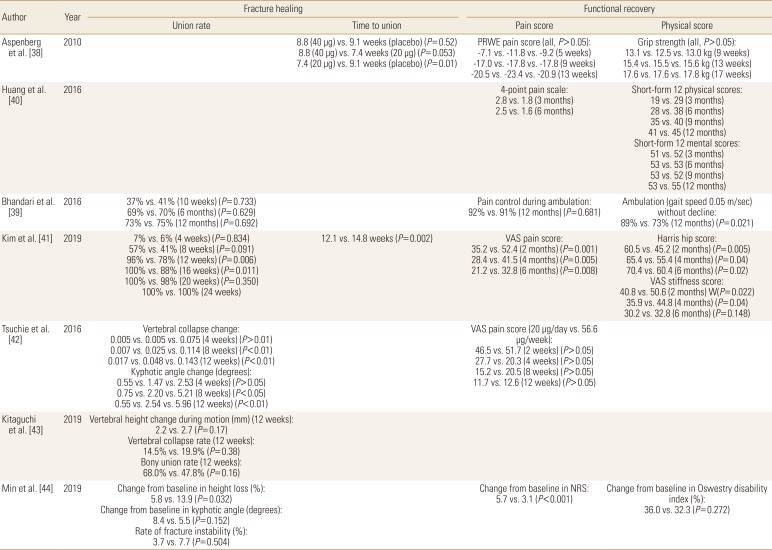
TPTD appeared to improve early callus formation after DRF.[36] However, the influence of TPTD to the healing of DRF has not been evaluated well.[37] Only one multicenter RPCT reported that the median time to union of non-surgically treated DRF was superior in TPTD treated patients by about 1 to 2 weeks compared with controls. However, improvement of pain and functional scores were not significantly different between these groups.[38]
The influence of TPTD to the hip fractures is still controversial. In one RPCT for patients with femoral neck fracture treated with internal fixation, the proportion of patients whose fractures healed or who required revision surgery did not significantly differ between the TPTD treated patients and placebo-treated controls. In addition, TPTD treatment did not improve radiographic signs of fracture healing or decrease pain compared with placebo treatment.[39] However, 2 retrospective studies reported findings that conflict with the previous study. Huang et al. [40] reported that TPTD treated patients showed better pain recovery and quality of life after internal fixation for intertrochanteric fracture. In a study of patients with intertrochanteric fracture who was treated with proximal femoral nail fixation, Kim et al. [41] reported that time to fracture union and pain and functional scores after 6 months following the procedure were superior in TPTD treated patients when compared to controls.
The influence of TPTD on osteoporotic vertebral fractures remains unclear. In 1 retrospective study, vertebral body collapse and local kyphotic angle change were significantly lower in TPTD treated patients with thoracolumbar spine fracture,[42] but those stability parameters were not significantly different between the groups in other 2 recent studies (Tables 4 and 5).[43,44]
SrR is a unique antiresorptive drug that may have anabolic properties.[7] It inhibits osteoclast differentiation and promotes osteoclast apoptosis. For anabolic effects, there are several controversies, but it is known that it activates pre-osteoblasts and replaces calcium with strontium, which leads to an increase in BMD.[45,46]
There have been a few animal studies investigating the impact of SrR on fracture healing. In osteoporotic ovariectomized rats, SrR significantly increased callus bone formation, maturity, and mineralization of fracture sites.[47,48] There have also been several clinical case reports with findings that support the beneficial effect of SrR on fracture healing and nonunion.[49,50] Recently, 1 RCT was performed in patients with wrist fracture to evaluate the efficacy of adding SrR to calcium and vitamin D supplementation in enhancing the fracture healing. All patients were older than 60 years and had undergone conservative treatment with manual reduction and cast application. The researchers concluded that SrR administered in the acute phase did not improve nor accelerate wrist fracture healing.[51] Except this, there are no other high level studies evaluating the influence of SrR on fracture healing.
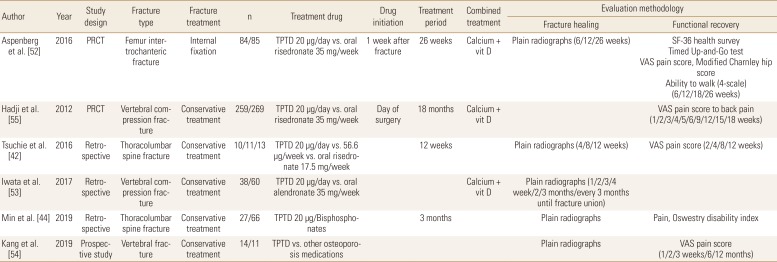
Recent widespread usage of TPTD in osteoporotic fracture patients make it possible to compare its role in fracture healing with other medications, especially BPs. Aspenberg et al. [52] compared TPTD and risedronate in patients with femur intertrochanteric fractures treated with internal fixation. TPTD was associated with less pain and a less time to complete the Timed Up-and-Go test between 6 and 26 weeks, compared with risedronate. However, other fracture-recovery outcomes including fracture union rate, time to union, and physical scores were similar between the groups.
Comparisons between TPTD and BPs were most commonly performed for patients with osteoporotic vertebral fractures. Tsuchie et al. [42] reported less vertebral collapse and kyphotic angle change in TPTD treated group, but Iwata et al. [53] found that fracture site stability parameters were not significantly different between the groups. In addition, Min et al. [44] reported that change of vertebral body height loss was favorable to TPTD treated patients, but change of local kyphosis and the rate of fracture instability were similar between the groups. For fracture site pain, 2 studies reported significantly less pain in TPTD treated patients at last follow-up,[42,44] 1 study found that TPTD treated patients had less pain, but the findings were not statistically significant,[54] and 1 study reported results according to the pain measurement methods (Tables 6 and 7).[55]
There were several limitations in this systematic review. First, we covered the representative osteoporotic fractures; wrist, hip, and spine fractures, for thorough and organized analysis. However, the effect of osteoporosis medications on the other fractures and atypical fracture have been studied and would be important future subjects. Second, collecting data from each study was done in objective manner, but comprehensive analysis and evaluation were done by authors and it would be a source of bias.
BPs administration did not influence on the fracture healing after DRF, hip fractures, vertebral fractures. Although evidence is still lacking, denosumab did not delay non-vertebral fracture healing, and there were no human studies about the influence of SERMs on fracture healing. TPTD showed shorter fracture healing time in DRF patients, while controversy in healing time, but better pain and functional outcomes in hip fractures. In vertebral fractures, TPTD had no evidence of shortening fracture healing time, but showed better improvement in fracture site pain. Considering no clinical data of negative fracture healing of the antiresorptive medication and the danger of subsequent fracture after initial osteoporotic fracture, there is no evidence to delay initiation of osteoporosis medications after fracture.
References
1. Khosla S, Cauley JA, Compston J, et al. Addressing the crisis in the treatment of osteoporosis: A path forward. J Bone Miner Res 2017;32:424-430.


2. Nakayama A, Major G, Holliday E, et al. Evidence of effectiveness of a fracture liaison service to reduce the re-fracture rate. Osteoporos Int 2016;27:873-879.


3. Sietsema DL, Araujo AB, Wang L, et al. The effectiveness of a private orthopaedic practice-based osteoporosis management service to reduce the risk of subsequent fractures. J Bone Joint Surg Am 2018;100:1819-1828.


4. Qaseem A, Snow V, Shekelle P, et al. Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2008;149:404-415.


6. Odvina CV, Zerwekh JE, Rao DS, et al. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 2005;90:1294-1301.


7. Hegde V, Jo JE, Andreopoulou P, et al. Effect of osteoporosis medications on fracture healing. Osteoporos Int 2016;27:861-871.


8. Amanat N, McDonald M, Godfrey C, et al. Optimal timing of a single dose of zoledronic acid to increase strength in rat fracture repair. J Bone Miner Res 2007;22:867-876.


9. McDonald MM, Dulai S, Godfrey C, et al. Bolus or weekly zoledronic acid administration does not delay endochondral fracture repair but weekly dosing enhances delays in hard callus remodeling. Bone 2008;43:653-662.


10. van der Poest Clement E, Patka P, Vandormael K, et al. The effect of alendronate on bone mass after distal forearm fracture. J Bone Miner Res 2000;15:586-593.


11. Rozental TD, Vazquez MA, Chacko AT, et al. Comparison of radiographic fracture healing in the distal radius for patients on and off bisphosphonate therapy. J Hand Surg Am 2009;34:595-602.


12. Shoji KE, Earp BE, Rozental TD. The effect of bisphosphonates on the clinical and radiographic outcomes of distal radius fractures in women. J Hand Surg Am 2018;43:115-122.


13. Gong HS, Song CH, Lee YH, et al. Early initiation of bisphosphonate does not affect healing and outcomes of volar plate fixation of osteoporotic distal radial fractures. J Bone Joint Surg Am 2012;94:1729-1736.


14. Uchiyama S, Itsubo T, Nakamura K, et al. Effect of early administration of alendronate after surgery for distal radial fragility fracture on radiological fracture healing time. Bone Joint J 2013;95-B:1544-1550.


15. Duckworth AD, McQueen MM, Tuck CE, et al. Effect of alendronic acid on fracture healing: A multicenter randomized placebo-controlled trial. J Bone Miner Res 2019;34:1025-1032.


16. Altintaş F, Ozkut AT, Beyzadeoğlu T, et al. The effect of risedronate treatment on bone turnover markers in patients with hip fracture. Acta Orthop Traumatol Turc 2007;41:132-135.

17. Cecilia D, Jódar E, Fernandez C, et al. Effect of alendronate in elderly patients after low trauma hip fracture repair. Osteoporos Int 2009;20:903-910.


18. Colón-Emeric C, Nordsletten L, Olson S, et al. Association between timing of zoledronic acid infusion and hip fracture healing. Osteoporos Int 2011;22:2329-2336.


19. Kim TY, Ha YC, Kang BJ, et al. Does early administration of bisphosphonate affect fracture healing in patients with intertrochanteric fractures? J Bone Joint Surg Br 2012;94:956-960.


20. Lim EJ, Kim JT, Kim CH, et al. Effect of preoperative bisphosphonate treatment on fracture healing after internal fixation treatment of intertrochanteric femoral fractures. Hip Pelvis 2019;31:75-81.



21. Ha KY, Park KS, Kim SI, et al. Does bisphosphonate-based anti-osteoporosis medication affect osteoporotic spinal fracture healing? Osteoporos Int 2016;27:483-488.


22. Goldhahn J, Feron JM, Kanis J, et al. Implications for fracture healing of current and new osteoporosis treatments: an ESCEO consensus paper. Calcif Tissue Int 2012;90:343-353.


23. Gerstenfeld LC, Sacks DJ, Pelis M, et al. Comparison of effects of the bisphosphonate alendronate versus the RANKL inhibitor denosumab on murine fracture healing. J Bone Miner Res 2009;24:196-208.

24. Adami S, Libanati C, Boonen S, et al. Denosumab treatment in postmenopausal women with osteoporosis does not interfere with fracture-healing: results from the FREEDOM trial. J Bone Joint Surg Am 2012;94:2113-2119.


25. Sahiner T, Aktan E, Kaleli B, et al. The effects of postmenopausal hormone replacement therapy on sympathetic skin response. Maturitas 1998;30:85-88.


26. Taranta A, Brama M, Teti A, et al. The selective estrogen receptor modulator raloxifene regulates osteoclast and osteoblast activity in vitro. Bone 2002;30:368-376.


27. Cao Y, Mori S, Mashiba T, et al. Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res 2002;17:2237-2246.


28. Beil FT, Barvencik F, Gebauer M, et al. Effects of estrogen on fracture healing in mice. J Trauma 2010;69:1259-1265.


29. Spiro AS, Khadem S, Jeschke A, et al. The SERM raloxifene improves diaphyseal fracture healing in mice. J Bone Miner Metab 2013;31:629-636.


30. Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 2000;21:115-137.

31. Friedl G, Turner RT, Evans GL, et al. Intermittent parathyroid hormone (PTH) treatment and age-dependent effects on rat cancellous bone and mineral metabolism. J Orthop Res 2007;25:1454-1464.


32. Kakar S, Einhorn TA, Vora S, et al. Enhanced chondrogenesis and Wnt signaling in PTH-treated fractures. J Bone Miner Res 2007;22:1903-1912.


33. Nakazawa T, Nakajima A, Shiomi K, et al. Effects of low-dose, intermittent treatment with recombinant human parathyroid hormone (1-34) on chondrogenesis in a model of experimental fracture healing. Bone 2005;37:711-719.


34. Manabe T, Mori S, Mashiba T, et al. Human parathyroid hormone (1-34) accelerates natural fracture healing process in the femoral osteotomy model of cynomolgus monkeys. Bone 2007;40:1475-1482.


35. Jørgensen NR, Schwarz P. Effects of anti-osteoporosis medications on fracture healing. Curr Osteoporos Rep 2011;9:149-155.


36. Aspenberg P, Johansson T. Teriparatide improves early callus formation in distal radial fractures. Acta Orthop 2010;81:234-236.



37. Shin YH, Gong HS. Recent update in the diagnosis and treatment of bone frailty in patients with a distal radius fracture. J Hand Surg Asian Pac Vol 2016;21:307-312.


38. Aspenberg P, Genant HK, Johansson T, et al. Teriparatide for acceleration of fracture repair in humans: a prospective, randomized, double-blind study of 102 postmenopausal women with distal radial fractures. J Bone Miner Res 2010;25:404-414.


39. Bhandari M, Jin L, See K, et al. Does teriparatide improve femoral neck fracture healing: Results from a randomized placebo-controlled trial. Clin Orthop Relat Res 2016;474:1234-1244.



40. Huang TW, Chuang PY, Lin SJ, et al. Teriparatide improves fracture healing and early functional recovery in treatment of osteoporotic intertrochanteric fractures. Medicine (Baltimore) 2016;95:e3626.



41. Kim SJ, Park HS, Lee DW, et al. Short-term daily teriparatide improve postoperative functional outcome and fracture healing in unstable intertrochanteric fractures. Injury 2019;50:1364-1370.


42. Tsuchie H, Miyakoshi N, Kasukawa Y, et al. The effect of teriparatide to alleviate pain and to prevent vertebral collapse after fresh osteoporotic vertebral fracture. J Bone Miner Metab 2016;34:86-91.


43. Kitaguchi K, Kashii M, Ebina K, et al. Effects of weekly teriparatide administration for vertebral stability and bony union in patients with acute osteoporotic vertebral fractures. Asian Spine J 2019;13:763-771.



44. Min HK, Ahn JH, Ha KY, et al. Effects of anti-osteoporosis medications on radiological and clinical results after acute osteoporotic spinal fractures: a retrospective analysis of prospectively designed study. Osteoporos Int 2019;30:2249-2256.


45. Sibai T, Morgan EF, Einhorn TA. Anabolic agents and bone quality. Clin Orthop Relat Res 2011;469:2215-2224.


46. Ohtori S, Inoue G, Orita S, et al. Comparison of teriparatide and bisphosphonate treatment to reduce pedicle screw loosening after lumbar spinal fusion surgery in postmenopausal women with osteoporosis from a bone quality perspective. Spine (Phila Pa 1976) 2013;38:E487-E492.


47. Ozturan KE, Demir B, Yucel I, et al. Effect of strontium ranelate on fracture healing in the osteoporotic rats. J Orthop Res 2011;29:138-142.


48. Li YF, Luo E, Feng G, et al. Systemic treatment with strontium ranelate promotes tibial fracture healing in ovariec tomized rats. Osteoporos Int 2010;21:1889-1897.


49. Tarantino U, Celi M, Saturnino L, et al. Strontium ranelate and bone healing: report of two cases. Clin Cases Miner Bone Metab 2010;7:65-68.


50. Alegre DN, Ribeiro C, Sousa C, et al. Possible benefits of strontium ranelate in complicated long bone fractures. Rheumatol Int 2012;32:439-443.


51. Scaglione M, Fabbri L, Casella F, et al. Strontium ranelate as an adjuvant for fracture healing: clinical, radiological, and ultrasound findings in a randomized controlled study on wrist fractures. Osteoporos Int 2016;27:211-218.


52. Aspenberg P, Malouf J, Tarantino U, et al. Effects of teriparatide compared with risedronate on recovery after pertrochanteric hip fracture: Results of a randomized, active-controlled, double-blind clinical trial at 26 weeks. J Bone Joint Surg Am 2016;98:1868-1878.



53. Iwata A, Kanayama M, Oha F, et al. Effect of teriparatide (rh-PTH 1-34) versus bisphosphonate on the healing of osteoporotic vertebral compression fracture: A retrospective comparative study. BMC Musculoskelet Disord 2017;18:148.



Table 2
Summary of published studies with bisphosphonates treatment in osteoporotic fracture healing (study protocol and methodology)
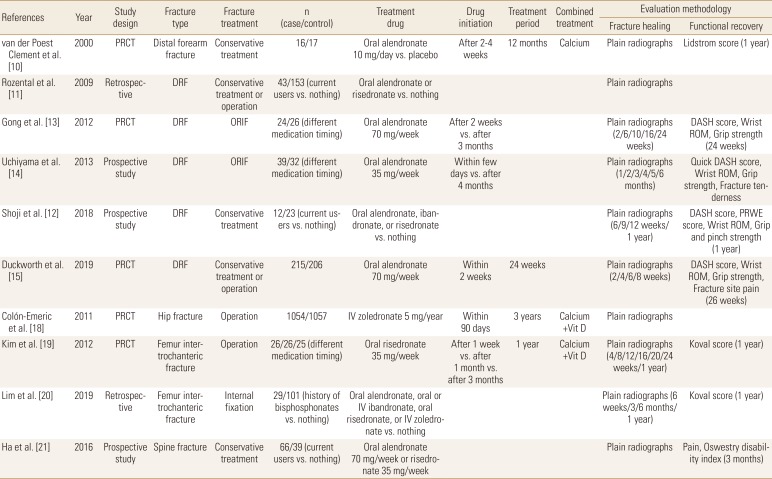
Table 3
Summary of published studies with bisphosphonates treatment in osteoporotic fracture healing (outcomes)

Table 4
Summary of the published studies with intermittent parathyroid hormone administration in osteoporotic fractures (study protocol and methodology)

Table 5
Summary of the published studies with intermittent parathyroid hormone administration in osteoporotic fractures (outcomes)
- TOOLS
-
METRICS

- Related articles
-
The Use of Osteoporosis Medications in Korea in 20082009 November;16(2)






 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print