|
 |
| jbm > Volume 26(1); 2019 > Article |
|
Abstract
Background
The effects of subclinical hyperthyroidism on bone mineral density (BMD) induced by thyroid-stimulating hormone (TSH) suppression therapy in patients with differentiated thyroid cancer (DTC) remains unclear. We conducted a meta-analysis to determine the influence of TSH suppression therapy on BMD.
Methods
We performed a systematic search to identify studies which included BMD measurement of femoral neck, total hip or lumbar spine in patients on TSH suppression therapy for DTC. Main outcome measures were difference of BMD of femoral neck, total hip or lumbar spine measured by dual energy X-ray absorptiometry between patients and controls.
Results
A systematic search yielded a total of 11 published controlled cross-sectional studies (including about 571 patients and 836 controls). TSH suppression therapy was associated with the lower BMD of total hip (weighted mean difference [WMD], −0.023; 95% confidence interval [CI], −0.047 to 0.000; P=0.050) and spine (WMD, −0.041; 95% CI, −0.057 to −0.026; P<0.001) in postmenopausal women with DTC, while it was not associated with that in premenopausal women and men with DTC.
Conclusions
Although the included studies were limited by small numbers, results suggested possible association between chronic TSH suppression therapy and the lower BMD of spine and total hip in postmenopausal women (but not in premenopausal women and men) with DTC. A large, well-designed study with long-term follow-up would provide further insight into the influence of TSH suppression therapy and loss of BMD.
Thyroid cancer is one of the most common endocrine malignancy.[1] Majority of thyroid cancer is differentiated thyroid cancer (DTC) raised from thyroid follicular epithelial cells, which are considered well-differentiated tumors, and have an overall excellent prognosis, with reported 10-year survival rates reaching 90%.[1,2,3] The excellent prognosis of DTC is because of a combination of the favorable biologic behavior of tumor cell and effective therapeutic modalities. The ‘standard’ treatment strategy for DTC includes surgery (near total/total thyroidectomy) followed by radioactive iodine (131-I) ablation of the surgical remnant and metastatic lesion, and long-term thyrotropin (TSH) suppression therapy.[3,4] DTC expresses thyroid-stimulating hormone (TSH) receptor on the cell membrane and TSH stimulates cell growth rate.[5] Thus the long-term suppression of TSH by supra-physiologic doses of L-thyroxine is used to treat patients with DTC with the purpose of decreasing the risk of cancer recurrence.[6,7,8,9,10]
However, it has been suggested that the long-term TSH suppression may be associated with potential undesired adverse effects of thyroxine on bone metabolism [11,12] as well as the major cardiovascular events [13,14,15,16,17,18] and atrial fibrillation,[19,20] because this represents in effect a state of chronic subclinical hyperthyroidism. Although normal euthyroid status during childhood and adolescence is required for acquisition of peak bone mass, overt hyperthyroidism is associated with an increased risk for osteoporosis.[21,22,23] The elevated level of thyroid hormone can excessively stimulate a bone turnover,[24] and shorten the bone remodeling cycle [25] which lead to consequent bone loss and decrease of bone mineral density (BMD). Therefore, patients who underwent TSH suppression therapy after thyroidectomy could be vulnerable to osteoporosis and decreased BMD.[19,26,27,28]
However, there is no consensus about the influence of long-term TSH suppression following thyroidectomy on BMD in patients with DTC, because of different study design (cross-sectional and longitudinal study), included patient groups (premenopausal and postmenopausal women, and men), methodology measuring BMD, area of interest for BMD (femoral neck and lumbar spine), and choice of outcome parameters (T-score, Z-score, and absolute value of BMD).[29,30,31,32]
Therefore, the purpose of this study was to determine whether TSH suppression therapy in patients with DTC influence BMD from the literature review and meta-analysis.
This study was exempted from Institutional Review Board review because it did not involve any human subjects.
This meta-analysis was conducted according to the updated Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) guidelines.[33] Searches of PubMed-Medline, EMBASE, and Cochrane Library were conducted by using key terms (“thyroid cancer or thyroidectomy” and “osteoporosis or osteoporotic fracture”) (Supplementary Appendix 1). The last search was conducted on September 26, 2018. Two authors (YJL and YKL) independently screened the titles and abstracts to identify studies on BMD in thyroid cancer. They also checked the reference lists of all potentially eligible studies and review papers to find out additional relevant publications.
Studies were screened and selected by all investigators on the basis of a priori criteria.
The inclusion criteria were (1) published as an original article in English; (2) included TSH suppression therapy in patients with DTC; (3) controlled cross-sectional studies (patients compared to a normal control group more or less carefully matched for age, sex, and menopausal status at least); (4) evaluated the BMD as primary outcome by using dual energy X-ray absorptiometry (DXA) in femoral neck, total hip or lumbar spine; and (5) available numerical data for both patients and controls (number of patients, mean and standard deviation of BMD according to the menopausal status).
Exclusion criteria were (1) cannot evaluate numerical data for patients with DTC; if the study included other conditions such as medullary cancer and toxic goiter; (2) not included TSH suppression therapy; (3) not available menopausal status; (4) measured BMD not by using DXA; (5) not reported value of BMD of femoral neck, total hip or lumbar spine; (6) not have normal control group; and (7) reviews and collection of abstracts for conference meeting.
Two authors (YJL and YKL) reviewed the retrieved full manuscripts to determine whether value of BMD after TSH suppression therapy in patients with DTC in femoral neck or lumbar spine had been reported.
The primary outcomes for the meta-analysis was the difference of BMD between patients with TSH suppression therapy after surgery for DTC and control group.
The studies were categorized according to gender and menopausal state and subgroup analysis undertaken accordingly (premenopausal women, postmenopausal women, and men).
For every eligible study, the following data were extracted and entered in a spread sheet by the 2 reviewers: the family name of the first author, year of publication, country, number of patients, mean duration of TSH suppression therapy, sample characteristics (age and gender), the mean value of BMD (g/cm2) in femoral neck or lumbar spine.
Two of the authors (YJL and YKL) independently evaluated the quality of all the studies, using Newcastle-Ottawa Scales.[34] This tool comprises three parameters: selection, comparability, and outcome. Each parameter consists of subcategorized questions: selection (a maximum of 4 stars), comparability (a maximum of 2 stars), and exposure or outcome (a maximum of 3 stars). We assessed the publication bias with Begg's funnel plot [35] and Egger's test [36].
We calculated the weighted mean difference (WMD) representing the magnitude of the difference between the comparative groups for each outcome, because all studies used the same outcome and unit of measurement (g/cm2).[37] WMD were computed separately for all available treatment and control groups for each study. We had used a fixed-effects or random-effects model depending on the results of heterogeneity to quantify the pooled effect size of the included studies (Values of P-value of less than 0.1 or an I2 value higher than 50% meant significant heterogeneity and a random-effects model should be applied). All analyses were performed using STATA (version 14.0; Stata Corp., College Station, TX, USA).
A primary search from the PubMed-Medline, EMBASE, and Cochrane Library, yielded 1,083 published articles. After duplicates removed, 889 articles were primarily screened by title and abstract. As a result, 71 articles were selected and reviewed for eligibility by full-text papers and a total of 11 cross-sectional studies fulfilling all inclusion criteria were included in the final analysis (Fig. 1).[11,38,39,40,41,42,43,44,45,46,47]
The results of the subgroup analyses according to gender and menopausal state were as follows.
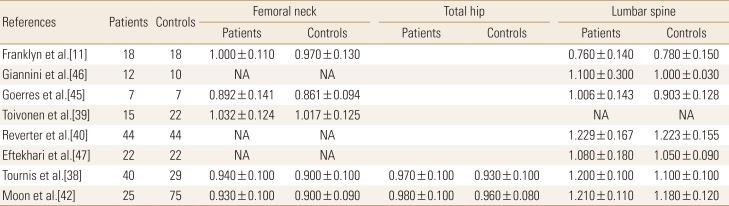
The effect of TSH suppression therapy on BMD in premenopausal women is described in 8 studies involving a total of 183 patients and 227 controls (Table 1). Femoral neck BMD (pooled WMD, 0.029; 95% confidence interval [CI], 0.005-0.054; P=0.020), and spine BMD (pooled WMD, 0.049; 95% CI, 0.022-0.076; P<0.001) were significantly higher in patients with TSH suppression therapy than control group, while total hip BMD (pooled WMD, 0.029; 95% CI, −0.003 to 0.061; P=0.076) did not differ significantly in premenopausal women (Fig. 2).
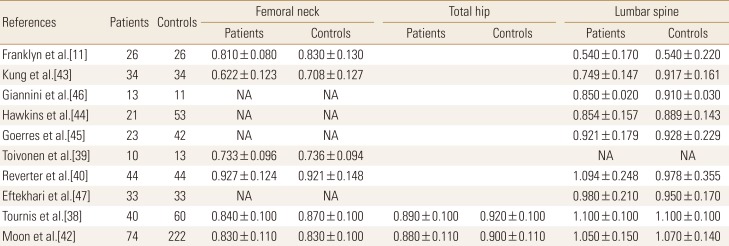
The effect of TSH suppression therapy in postmenopausal women was investigated in 10 studies involving a total 318 patients and 538 controls (Table 2). Total hip BMD (pooled WMD, −0.023; 95% CI, −0.047 to 0.000; P=0.050), and spine BMD (pooled WMD, −0.041; 95% CI, −0.057 to −0.026; P<0.001) and were significantly lower in patients with TSH suppression therapy than control group, while femoral neck BMD (pooled WMD, −0.016; 95% CI −0.035 to 0.002; P=0.084) did not differ significantly (Fig. 3).
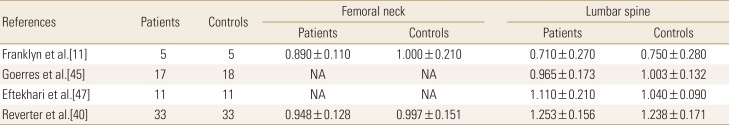
Four studies involving a total 66 patients and 67 controls were identified for analysis addressed the effects of TSH suppression therapy on bone metabolism in men in a cross-sectional study design (Table 3). No study showed a significant difference of BMD between patients and controls. Femoral neck BMD (pooled WMD, −0.055; 95% CI, −0.119 to 0.009; P=0.094), and spine BMD (pooled WMD, 0.007; 95% CI, −0.049 to 0.063; P=0.803) did not differ significantly in men (Fig. 4).
In terms of the methodological quality, the mean value of the awarded star was 5.3 (5 stars [2 studies], 6 stars [9 studies]; Supplementary Table 1). The Begg's funnel plot was not asymmetrical, and P-value for bias were not significant in all outcomes (Fig. 5).
The clinical implications of long-term TSH suppression therapy on bone are critical, largely because of the favorable prognosis of DTC and long-term survival of patients with DTC.[10] Subclinical thyroid dysfunction has been known to be associated with increased risk of hip fracture,[48] but the influence of chronic subclinical hyperthyroidism, TSH suppression therapy, on decreased BMD in patients who underwent thyroidectomy for DTC remain controversial. Our aim was to review the literature on the effects of TSH suppression therapy on BMD in patients with DTC.
Although there have been many studies on this issue, each study had a different outcome as well as different tools for measurement of BMD. Many previous studies have used single or dual photon absorptiometry, not DXA.[49,50,51,52,53,54]
The majority of studies reported no effect of TSH suppression therapy on BMD in men and premenopausal women.[40,41,42] Our meta-analysis also showed no influence of TSH suppression therapy on BMD in men and premenopausal women. On the other hands, the influence in postmenopausal women remain unclear. Our meta-analysis showed the TSH suppression therapy was associated with lower BMD of total hip and spine in postmenopausal women. We could not determine conclusively this issue, because of too small number of the included studies and pooled patients, although we performed a meta-analysis.
However, some large population-based cohort studies showed the increased risk of osteoporosis and osteoporotic fracture such as hip fracture and vertebral fracture.[55,56] Lin et al.[56] compared the risk of osteoporosis and osteoporotic fracture among 9,398 thyroid cancer patients with levothyroxine use (n=538), those (n=8,860) without levothyroxine use and propensity-score-matched controls (n=9,398). They showed that the incidence of osteoporosis and osteoporotic fracture in the thyroid cancer patients (8.69/1,000 person-years) was higher than that in the non-thyroid-cancer cohort (6.60/1,000 person-years) (adjusted hazard ratio, 1.39; 95% CI, 1.22-1.58). They also presented that long duration of levothyroxine use, and high cumulative dose of levothyroxine were significantly associated with an increased risk of osteoporosis in thyroid cancer patients following thyroidectomy.[56]
Based on our meta-analysis of available data, we identified postmenopausal women with DTC receiving TSH suppression therapy as a risk group for bone loss. Considering menopause as the most important risk factor of osteoporosis,[57] the discrepancy in results between premenopausal and postmenopausal women might be explained by a different susceptibility according to menopausal status.
The present study has a limitation. The cumulative sample size was not very large because most of the studies included relatively few patients. Thus the results of subgroup analysis are also very limited due to small sample size. But we used the weighted effect sizes by including studies only had used DXA, the effect size is easily interpreted from a clinical point of view.
Overall, although studies were limited by small numbers, results suggested possible association between chronic TSH suppression therapy and the higher risk of low BMD in postmenopausal women with TSH suppression therapy. And, it is clear that larger-scale, better-designed studies that report effects of TSH suppression therapy on BMD are needed in the future to determine the influence of TSH suppression therapy on risk of osteoporotic fracture in DTC.
ACKNOWLEDGMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant no. HI18C0284).
SUPPLEMENTARY MATERIALS
Supplementary Appendix 1
The search strategy that details the searching process of relevant clinical study selection
Supplementary Table 1
The quality assessment of each study according to the Newcastle-Ottawa Scales
References
1. Lim H, Devesa SS, Sosa JA, et al. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA 2017;317:1338-1348.



2. Mirian C, Grønhøj C, Jensen DH, et al. Trends in thyroid cancer: retrospective analysis of incidence and survival in Denmark 1980-2014. Cancer Epidemiol 2018;55:81-87.


3. Tam S, Boonsripitayanon M, Amit M, et al. Survival in differentiated thyroid cancer: comparing the AJCC cancer staging seventh and eighth editions. Thyroid 2018;28:1301-1310.


4. Mazzaferri EL, Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab 2001;86:1447-1463.


6. Haugen BR. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: what is new and what has changed? Cancer 2017;123:372-381.


7. Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 1994;97:418-428.


8. Goretzki PE, Frilling A, Simon D, et al. Growth regulation of normal thyroids and thyroid tumors in man. Recent Results Cancer Res 1990;118:48-63.

9. Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid 1998;8:737-744.


10. McGriff NJ, Csako G, Gourgiotis L, et al. Effects of thyroid hormone suppression therapy on adverse clinical outcomes in thyroid cancer. Ann Med 2002;34:554-564.


11. Franklyn JA, Betteridge J, Daykin J, et al. Long-term thyroxine treatment and bone mineral density. Lancet 1992;340:9-13.


12. Quan ML, Pasieka JL, Rorstad O. Bone mineral density in well-differentiated thyroid cancer patients treated with suppressive thyroxine: a systematic overview of the literature. J Surg Oncol 2002;79:62-69.


13. Biondi B, Palmieri EA, Fazio S, et al. Endogenous subclinical hyperthyroidism affects quality of life and cardiac morphology and function in young and middle-aged patients. J Clin Endocrinol Metab 2000;85:4701-4705.

14. Biondi B, Palmieri EA, Lombardi G, et al. Effects of thyroid hormone on cardiac function: the relative importance of heart rate, loading conditions, and myocardial contractility in the regulation of cardiac performance in human hyperthyroidism. J Clin Endocrinol Metab 2002;87:968-974.


15. Napoli R, Biondi B, Guardasole V, et al. Impact of hyperthyroidism and its correction on vascular reactivity in humans. Circulation 2001;104:3076-3080.


16. Sgarbi JA, Villaça FG, Garbeline B, et al. The effects of early antithyroid therapy for endogenous subclinical hyperthyroidism in clinical and heart abnormalities. J Clin Endocrinol Metab 2003;88:1672-1677.


17. Smit JW, Eustatia-Rutten CF, Corssmit EP, et al. Reversible diastolic dysfunction after long-term exogenous subclinical hyperthyroidism: a randomized, placebo-controlled study. J Clin Endocrinol Metab 2005;90:6041-6047.


18. Schlumberger M, Pacini F, Wiersinga WM, et al. Follow-up and management of differentiated thyroid carcinoma: a European perspective in clinical practice. Eur J Endocrinol 2004;151:539-548.


19. Rosario PW, Carvalho M, Calsolari MR. Symptoms of thyrotoxicosis, bone metabolism and occult atrial fibrillation in older women with mild endogenous subclinical hyperthyroidism. Clin Endocrinol (Oxf) 2016;85:132-136.


20. Karunakaran P, Maharajan C, Chockalingam R, et al. The effect of total thyroidectomy on the recovery of bone mineral density in subjects with hyperthyroidism. Surgery 2019;165:80-84.


21. Greenspan SL, Greenspan FS. The effect of thyroid hormone on skeletal integrity. Ann Intern Med 1999;130:750-758.


22. Tuchendler D, Bolanowski M. The influence of thyroid dysfunction on bone metabolism. Thyroid Res 2014;7:12




24. Eriksen EF, Mosekilde L, Melsen F. Trabecular bone remodeling and bone balance in hyperthyroidism. Bone 1985;6:421-428.


25. Mosekilde L, Melsen F, Bagger JP, et al. Bone changes in hyperthyroidism: interrelationships between bone morphometry, thyroid function and calcium-phosphorus metabolism. Acta Endocrinol (Copenh) 1977;85:515-525.


26. Abrahamsen B, Jørgensen HL, Laulund AS, et al. The excess risk of major osteoporotic fractures in hypothyroidism is driven by cumulative hyperthyroid as opposed to hypothyroid time: an observational register-based time-resolved cohort analysis. J Bone Miner Res 2015;30:898-905.


27. Gürlek A, Gedik O. Effect of endogenous subclinical hyperthyroidism on bone metabolism and bone mineral density in premenopausal women. Thyroid 1999;9:539-543.


28. Kim CW, Hong S, Oh SH, et al. Change of bone mineral density and biochemical markers of bone turnover in patients on suppressive levothyroxine therapy for differentiated thyroid carcinoma. J Bone Metab 2015;22:135-141.



29. Stěpán JJ, Límanová Z. Biochemical assessment of bone loss in patients on long-term thyroid hormone treatment. Bone Miner 1992;17:377-388.


30. Jódar E, Martínez-Díaz-Guerra G, Azriel S, et al. Bone mineral density in male patients with L-thyroxine suppressive therapy and Graves disease. Calcif Tissue Int 2001;69:84-87.



31. Heijckmann AC, Huijberts MS, Geusens P, et al. Hip bone mineral density, bone turnover and risk of fracture in patients on long-term suppressive L-thyroxine therapy for differentiated thyroid carcinoma. Eur J Endocrinol 2005;153:23-29.


32. Jódar E, Begoña López M, García L, et al. Bone changes in pre- and postmenopausal women with thyroid cancer on levothyroxine therapy: evolution of axial and appendicular bone mass. Osteoporos Int 1998;8:311-316.


33. Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647


34. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-605.



35. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-1101.


36. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-634.

37. Becker BJ. Synthesizing standardized mean-change measures. Br J Math Stat Psychol 1988;41:257-278.

38. Tournis S, Antoniou JD, Liakou CG, et al. Volumetric bone mineral density and bone geometry assessed by peripheral quantitative computed tomography in women with differentiated thyroid cancer under TSH suppression. Clin Endocrinol (Oxf) 2015;82:197-204.


39. Toivonen J, Tähtelä R, Laitinen K, et al. Markers of bone turnover in patients with differentiated thyroid cancer with and following withdrawal of thyroxine suppressive therapy. Eur J Endocrinol 1998;138:667-673.


40. Reverter JL, Holgado S, Alonso N, et al. Lack of deleterious effect on bone mineral density of long-term thyroxine suppressive therapy for differentiated thyroid carcinoma. Endocr Relat Cancer 2005;12:973-981.


41. Reverter JL, Colomé E, Holgado S, et al. Bone mineral density and bone fracture in male patients receiving long-term suppressive levothyroxine treatment for differentiated thyroid carcinoma. Endocrine 2010;37:467-472.



42. Moon JH, Jung KY, Kim KM, et al. The effect of thyroid stimulating hormone suppressive therapy on bone geometry in the hip area of patients with differentiated thyroid carcinoma. Bone 2016;83:104-110.


43. Kung AW, Lorentz T, Tam SC. Thyroxine suppressive therapy decreases bone mineral density in post-menopausal women. Clin Endocrinol (Oxf) 1993;39:535-540.


44. Hawkins F, Rigopoulou D, Papapietro K, et al. Spinal bone mass after long-term treatment with L-thyroxine in postmenopausal women with thyroid cancer and chronic lymphocytic thyroiditis. Calcif Tissue Int 1994;54:16-19.



45. Goerres G, Theiler R, Müller-Brand J. Interfemur variation of bone mineral density in patients receiving high-dose thyroxin therapy. Calcif Tissue Int 1998;63:98-101.



46. Giannini S, Nobile M, Sartori L, et al. Bone density and mineral metabolism in thyroidectomized patients treated with long-term L-thyroxine. Clin Sci (Lond) 1994;87:593-597.



47. Eftekhari M, Asadollahi A, Beiki D, et al. The long term effect of levothyroxine on bone mineral density in patients with well differentiated thyroid carcinoma after treatment. Hell J Nucl Med 2008;11:160-163.

48. Lee JS, Buzková P, Fink HA, et al. Subclinical thyroid dysfunction and incident hip fracture in older adults. Arch Intern Med 2010;170:1876-1883.



49. Stall GM, Harris S, Sokoll LJ, et al. Accelerated bone loss in hypothyroid patients overtreated with L-thyroxine. Ann Intern Med 1990;113:265-269.


50. Adlin EV, Maurer AH, Marks AD, et al. Bone mineral density in postmenopausal women treated with L-thyroxine. Am J Med 1991;90:360-366.


51. Greenspan SL, Greenspan FS, Resnick NM, et al. Skeletal integrity in premenopausal and postmenopausal women receiving long-term L-thyroxine therapy. Am J Med 1991;91:5-14.


52. Paul TL, Kerrigan J, Kelly AM, et al. Long-term L-thyroxine therapy is associated with decreased hip bone density in premenopausal women. JAMA 1988;259:3137-3141.

53. Recker DP, Shapiro B. The effect of thyroidectomy on bone mineral content in perimenopausal women. Thyroidology 1989;1:59-65.

54. Ross DS, Neer RM, Ridgway EC, et al. Subclinical hyperthyroidism and reduced bone density as a possible result of prolonged suppression of the pituitary-thyroid axis with L-thyroxine. Am J Med 1987;82:1167-1170.

55. Melton LJ 3rd, Ardila E, Crowson CS, et al. Fractures following thyroidectomy in women: a population-based cohort study. Bone 2000;27:695-700.


Fig. 1
Preferred Reporting Items for Systematic review and Meta-analysis flow diagram details the process of relevant study selection. DTC, differentiated thyroid cancer; TSH, thyroid-stimulating hormone; DXA, dual-energy X-ray absorptiometry; BMD, bone mineral density.
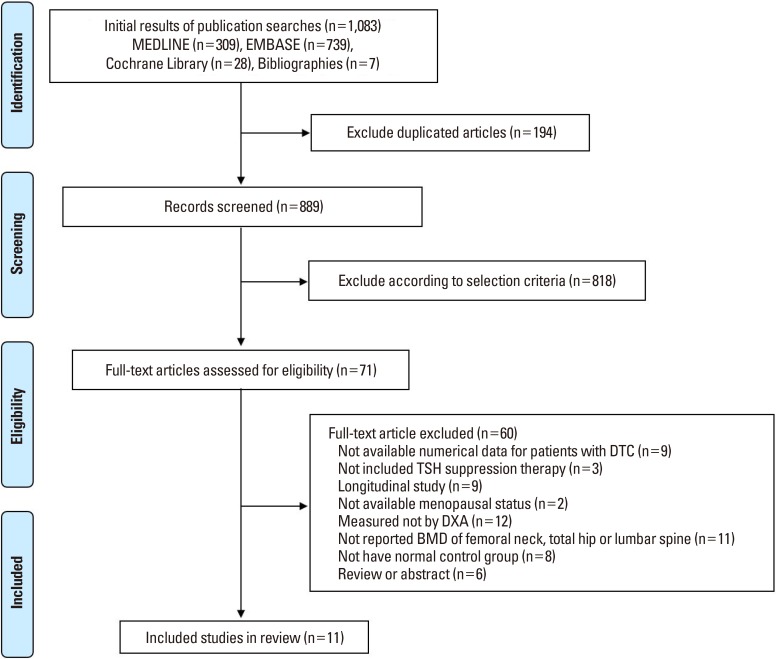
Fig. 2
Forest plot of effect of thyroid-stimulating hormone suppression therapy on femoral neck, total hip, and lumbar spine bone mineral density in premenopausal women with differentiated thyroid cancer determined by fixed effects meta-analysis. Effect sizes are indicated as Hedges' g standardized mean differences and 95% confidence interval (CI). WMD, weighted mean difference.
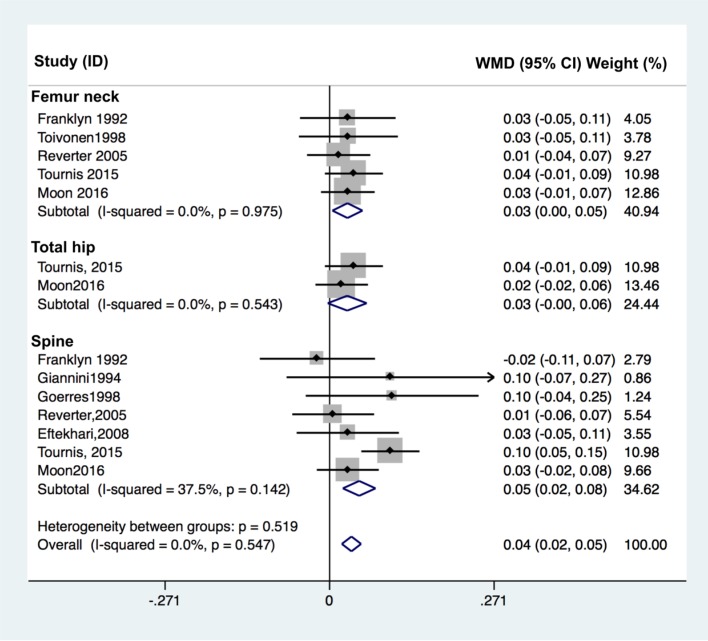
Fig. 3
Forest plot of effect of thyroid-stimulating hormone suppression therapy on femoral neck, total hip, and lumbar spine bone mineral density in postmenopausal women with differentiated thyroid cancer determined by fixed effects meta-analysis. Effect sizes are indicated as Hedges' g standardized mean differences and 95% confidence interval (CI). WMD, weighted mean difference.

Fig. 4
Forest plot of effect of thyroid-stimulating hormone suppression therapy on femoral neck and lumbar spine bone mineral density in men with differentiated thyroid cancer determined by fixed effects meta-analysis. Effect sizes are indicated as Hedges' g standardized mean differences and 95% confidence interval (CI). WMD, weighted mean difference.

Fig. 5
The Begg's funnel plot and P-value by Egger's test shows publication bias of femoral neck, total hip, and lumbar spine bone mineral density in each group. (A-C) Premenopausal women, (D-F) postmenopausal women, and (G, H) men. WMD, weighted mean difference.
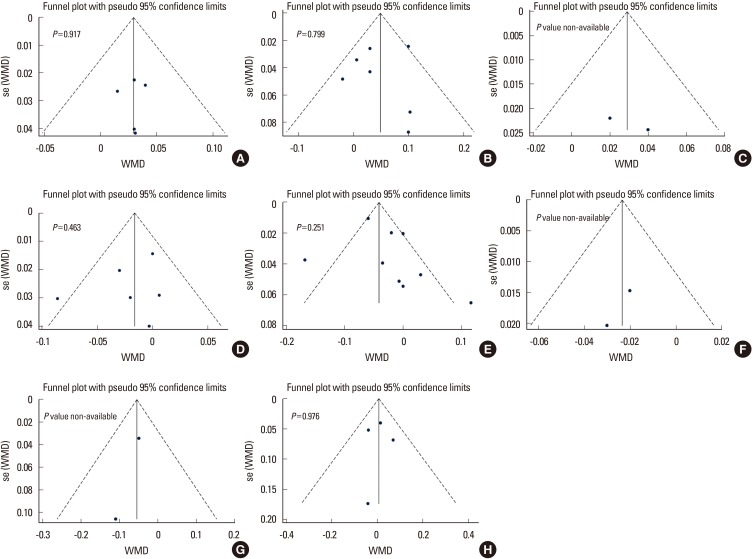
Table 1
Bone mineral density of thyroid-stimulating hormone suppression therapy group and control group in premenopausal women



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Supplement1
Supplement1 Print
Print