|
 |
| jbm > Volume 26(3); 2019 > Article |
|
Abstract
Background
The molecular pathways of how endocrine disruptors affect bone mineral density (BMD) and bone remodeling are still unclear. The purpose of this experimental study is to determine the effects of di(2-ethylhexyl)phthalate (DEHP) on bone metabolism in ovariectomized mice.
Methods
Twenty-six-month-old female CD-1 mice were divided into 4 groups: control, low-dose DEHP, high-dose DEHP, and estrogen groups (n=5, each group). All mice were subjected to ovariectomy for the induction of artificial menopause and then exposed to corn oil, DEHP, and estrogen for 2 months. Micro-computed tomography (Micro-CT) of the bone and analysis of blood samples for bone markers were performed to observe the changes in bone metabolism.
Results
Osteocalcin level was decreased in the control, low-dose and high-dose DEHP group, the reduction width was greater in the high-dose DEHP group (−0.219 ng/mL) than control group (−0.077 ng/mL, P<0.05). C-terminal telopeptide of type I collagen level was increased in the control, low-dose and high-dose DEHP group, the increase range of low-dose DEHP group (0.329 ng/mL) showed greater than control group (0.093 ng/mL, P<0.05). Micro-CT analysis revealed that the BMD was significantly lower in the high-dose DEHP group (19.8×10−2 g/cm3) than control group (27.2×10−2 g/cm3, P<0.05). The structure model index was significantly higher in the high-dose DEHP group (2.737) than low-dose DEHP group (2.648) and estrogen group (2.63, P<0.05). It means the progression of osteoporosis in the high-dose DEHP group.
Osteoporosis is a disease with various causes; estrogen abnormality, thyroid hormone abnormality, calcium (Ca) metabolic disorder, steroid use, aging, and lack of exercise influence its occurrence. It is a disease where bone fractures develop easily due to the weakening of bone strength, as the overall bone metabolism declines. The balance between osteoclasts, which metabolize Ca by absorbing the bone, and the osteoblasts, which creates bone, also has a significant influence on its occurrence.
Endocrine disruptors, which are known to respond to estrogen receptors due to their structural similarity with estrogen, initiate the structural change of estrogen receptors, and act on several post-receptor responses associated with estrogen.[1] Due to such characteristics, the associations between diseases that develop in a variety of target organs affected by estrogen are being discovered, of which the typical examples are breast cancer and other obstetric and gynecological diseases caused by estrogen imbalance. Among various previous studies on bone mineral density (BMD) and endocrine disruptors, exposure to estrogen at the time of birth has been reported to have a significant effect on adult BMD, which is related not only to the duration of exposure but also to the concentration of estrogen.[2,3] In addition, there are studies reporting that exposure to environmental hormones during pregnancy and lactation affects BMD and bone remodeling.[4] In animal studies, phthalates have been observed to have an inhibitory effect on osteoblasts in mice, thereby affecting BMD, along with weak estrogenic and antiestrogenic activities, and anti-androgenic activity.[5,6,7,8]
The molecular pathways of how endocrine disruptors affect BMD and bone remodeling are still unknown. However, in addition to their previously identified effects on estrogen biosynthesis, estrogen metabolism and receptors, studies have also reported their effects on genes involved in the receptor sub-pathways or other genes that regulate osteocyte differentiation.[9] In our previous study, mice exposed to di(2-ethylhexyl)phthalate (DEHP) at prenatal and lactation period showed an abnormal amplification of genes related with bone remodeling in the adult stage.[10] Considering that menopausal osteoporosis is caused by the decline in estrogen levels, the possibility of endocrine disruptors having similar functions as estrogen, i.e., affecting BMD after menopause should be fully considered. In a previous study by Min and Min,[11] which investigated urinary phthalate metabolites and BMD in elderly women using the US National Health Survey data, it was reported that the higher the concentration of phthalate metabolites, the lower the BMD and the higher the incidence of osteoporosis.
The purpose of this experimental study is to determine the effects of DEHP on bone health in ovariectomized mice. Micro-computed tomography (Micro-CT) of the bone, and analysis of blood samples for bone markers were performed to observe the changes in bone metabolism.
Female adult CD-1 (Crl:CD-1 [ICR] BR) mice were obtained from Orient Bio Inc. (Seongnam, Korea) and raised at the animal lab of the College of Medicine, Catholic University of Korea. In each cage, four mice were raised on an aspen bedding (Tapvei, Paekna, Estonia) under controlled lighting (12 hr light/12 hr dark); appropriate conditions of temperature (22±5℃) and humidity (50%±10%) were maintained. The mice were fed with Teklad Global 18% Protein Rodent Diet (Harlan Laboratories Inc., Madison, WI, USA), and sterilized tap water was supplied through polycarbonate bottles. Animal breeding and study protocols were performed in compliance with the Laboratory Animals Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the Guidelines and Policies for Rodent Experiments, and were approved by the Institutional Animal Care and Use Committee (IACUC) of the School of Medicine, Catholic University of Korea (Approval no. CUMC-2014-0083-02). All animals were raised in humanitarian settings, and efforts were taken to minimize pain.
Twenty-six-month-old female CD-1 mice were divided into 4 groups: the control, low-dose DEHP treatment, high-dose DEHP treatment, and estrogen treatment groups; each group contained 5 mice. To prevent infection and reduce pain, gentamicin (5 mg/kg) and ketoprofen (5 mg/kg) were administered via subcutaneous injection once prior to surgery and for 3 days after surgery. The animals received inhalation anesthesia with isoflurane (1.5%) and were fixed to the anesthesia machine. An electric shaver was used to shave the surgical area, which was disinfected with alcohol. An incision was made at the middle abdominal region, and the ovaries at both sides were checked and then removed.
Ovariectomized mice were exposed to the following concentrations of DEHP and estrogen for 2 months after surgery; the control group received corn oil (0.15 mL/wk) through subcutaneous injection, low-dose DEHP treatment group received 35 µg/kg/wk (5 µg/kg/d) DEHP dissolved in 0.15 mL corn oil once a week via subcutaneous injection, high-dose DEHP treatment group received 350 µg/kg/wk (50 µg/kg/d) DEHP dissolved in 0.15 mL corn oil once a week via subcutaneous injection, and estrogen treatment group received conjugated equine estrogen (Premarin 0.3 mg/kg/day) once a day via per oral route. In the control and low-dose DEHP and high-dose DEHP treatment groups, venous blood was collected (0.3 mL) through the retro-orbital plexus once a month after ovariectomy to test for 2 types of bone markers (osteocalcin, C-terminal telopeptide of type I collagen [CTX-1]), and serum Ca, phosphorus (P), alkaline phosphatase (ALK-P), and magnesium (Mg).
A specific sandwich enzyme-linked immunosorbent assay (ELISA) was used to quantify CTX-1 (MyBioSource, Inc., San Diego, CA, USA; MB703094) and Gla-Osteocalcin (Takara Bio Inc., Otsu, Japan; MK127) in mouse serum. Aliquots of mouse serum samples were each tested in triplicate at several dilutions and compared to reference standards of CTX-1 and Gla-Osteocalcin. The concentration of CTX-1 and Gla-Osteocalcin was measured using ELISA kit according to the manufacture's protocol.
Mouse Gla-Osteocalcin High Sensitive EIA Kit (TAKARA MK127); Prepare reagents and samples (100 µL each) in a separate 96 well plate in advance so that they can be added to the Antibody Coated Microtiterplate quickly (within 5 min) using an 8-channel pipette or similar apparatus. Perform this reaction at room temperature (20-30℃) for 1 hr; incubation at 37℃ may compromise antigenicity (First reaction). Discard reaction mixtures, followed by 3 washes with Washing Buffer. Then add 100 µL of the POD-labeled Antibody Solution per well using an 8-channel pipette and allow to react for 1 hr at room temperature (20-30℃) (Second reaction). Discard reaction mixtures, followed by 4 washes with Washing Buffer. Then add 100 µL of Substrate Solution (TMBZ) per well using an 8-channel pipette and allow to react at room temperature (20-30℃) for 10 to 15 min (Third reaction). Add 100 µL of Stop Solution to each well to stop the reaction in the same order as for Substrate Solution (TMBZ). Then mix well. Use distilled water as a control to make zero adjustment and measure absorbance at 450 nm.
Mouse cross linked CTX-1 ELISA Kit (mybiosource MB703094); Prepare all reagents and samples as directed in the previous sections. Determine the number of wells to be used and put any remaining wells and the desiccant back into the pouch and seal the ziploc, store unused wells at 4℃. Set a Blank well without any solution. Add 100 µL of Standard or Sample per well. Standard need test in duplicate. Add 100 µL of HRP-conjugate to each well (not to Blank well), then 100 µL Antibody to each well. Mix well and then incubate for 1 hr at 37℃. Aspirate each well and wash, repeating the process 2 times for a total of 3 washes. Wash by filling each well with Wash Buffer (200 µL) using a squirt bottle, multi-channel pipette, manifold dispenser and let it stand for 10 sec, complete removal of liquid at each step is essential to good performance. After the last wash, remove any remaining Wash Buffer by aspirating ordecanting. Invert the plate and blot it against clean paper towels.
Add 50 µL of Substrate A and 50 µL of Substrate B to each well, mix well. Incubate for 15 min at 37℃. Keeping the plate away from drafts and other temperature fluctuations in the dark. Add 50 µL of Stop Solution to each well, gently tap the plate to ensure thorough mixing. Determine the optical density of each well within 10 min, using a microplate reader set to 450 nm.
All mice were sacrificed four months after surgery using carbon dioxide, the tibia samples were fixed with 70% ethanol. BMD, microstructures (structural thickness, structural separation, structural linear density), structure model index (SMI) of the tibia head were analyzed by Micro-CT (A SkyScan 1,176 instrument; Bruker microCT, Kontich, Belgium). Micro-CT analysis was carried out by SecondAnalysis corporation (Korea). CT Analysis and CT Volume programs (Bruker microCT) were used to obtain images and analyze data. For analyze data, we used Excel (Microsoft office 2017), paired t-test (P<0.05). Statistical comparisons were performed using student's t-test, and data were regarded as being significant when P<0.05.
Osteocalcin is a well-known bone formation marker. Level of osteocalcin showed increase in estrogen group (3.021 ng/mL and 3.11 ng/mL, at 1-2 months), but showed decrease in control group (3.355 ng/mL and 3.278 ng/mL), low-dose DEHP group (3.478 ng/mL and 3.39 ng/mL) and high-dose DEHP group (3.194 ng/mL and 2.975 ng/mL) (Fig. 1). The calculated subtraction value (2 months value minus 1 month value) is statistically greater in the high-dose DEHP group (−0.219 ng/mL) than the control group (−0.077 ng/mL, P<0.05). These data means bone formation was promoted in the estrogen treated group, but in the other groups, bone formation was decreased, especially in high-dose DEHP group.
In bone physiology, the CTX-1 is a telopeptide that can be used as a bone resorption biomarker in the serum to measure the rate of bone turnover. Increased CTX-1 means higher bone resorption status means higher bone turnover status. Serum CTX assay shows greater utility for assessing efficacy of antiresorptive treatment. Serum CTX-1 levels showed a decrease in the estrogen treatment group (1.336 ng/mL and 1.134 ng/mL, at 1 and 2 months), while an increase in the control (1.384 ng/mL and 1.477 ng/mL), low-dose DEHP (1.079 ng/mL and 1.408 ng/mL), and high-dose DEHP groups (1.406 ng/mL and 1.533 ng/mL) (Fig. 2). These data means bone resorption was inhibited in estrogen treatment group, but not in the other groups. Additionally, the calculated subtraction value (2 months value minus 1 month value) of low-dose DEHP treatment groups (0.329 ng/mL) showed greater than the control group (0.093 ng/mL, P<0.05).
Biochemical assays were performed using samples from the control, low-dose DEHP, and high-dose DEHP groups. There was no significant difference in the serum Ca levels and serum Mg levels between control and treatment groups. Serum P level in the high-dose DEHP group showed significant decrease (8.4 mg/dL and 6.8 mg/dL, at 1 and 4 months) than control group (6.9 mg/dL and 7.5 mg/dL), and the serum ALK-P levels were decreased to a greater extent in the low-dose DEHP group (100 mEq/L and 149 mEq/L, at 1 and 4 months) and high-dose DEHP groups (107 mEq/L and 132 mEq/L) than in the control group (147 mEq/L and 197 mEq/L, P<0.05) (Fig. 3).
After 4 months from ovariectomy, the BMD was significantly lower in the high-dose DEHP group (19.8×10−2 g/cm3) and significantly higher in the estrogen treatment group (30.9×10−2 g/cm3) than control group (27.2×10−2 g/cm3, P<0.05) (Fig. 4).
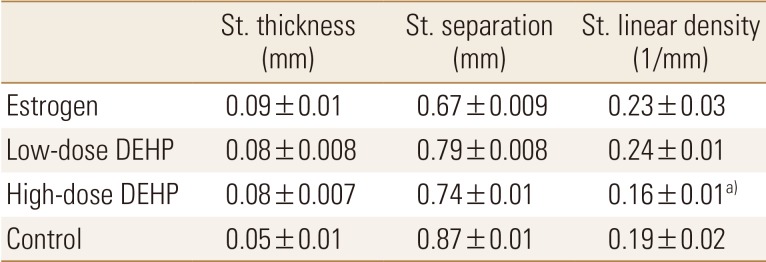
In the microstructural analysis using Micro-CT, structural thickness, structural separation, and structural linear density of tibia head were calculated. The structural linear density was significantly lower in the high-dose DEHP group (0.16±0.01/mm) than in the estrogen treatment (0.23±0.03/mm) and low-dose DEHP (0.24±0.01/mm) groups (P<0.05). No other significant structural differences were observed among the four groups (Table 1).
After the microstructural analysis using Micro-CT, trabecular area was also identified with yellow color (Fig. 5). Trabecular area is more prominent in estrogen treatment group. In high-dose DEHP group, trabecular area is smaller than the others (estrogen treatment, control, and low-dose DEHP groups).
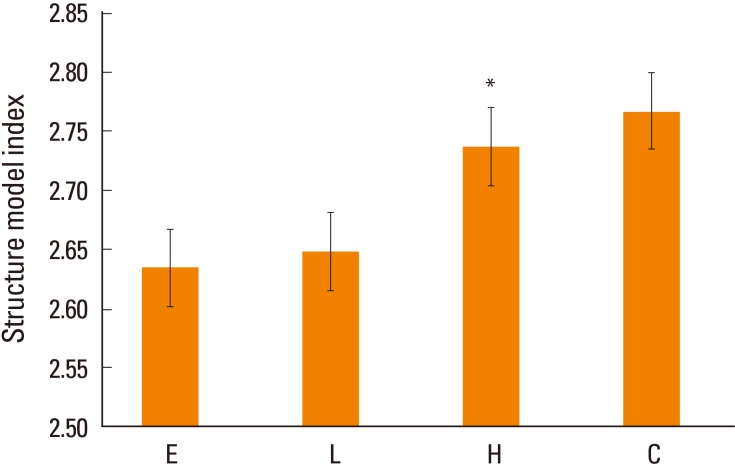
SMI suggests that the shape of the bone is dominant, either bar-like (score 3) or plate-like (score 0), and higher SMI means predominate bar shape, which means that the bone strength is weakened. SMI was significantly higher in the high-dose DEHP group (2.737) than in the estrogen treatment (2.63) and low-dose DEHP group (2.648, P<0.05). No significant differences were observed in the high-dose DEHP group and control group (2.767) (Fig. 6).
Osteoporosis is a major disease that increases mortality and the prevalence of the elderly in an aging society. In addition to age and menopause, there are various factors that increase the incidence of osteoporosis, including amount of exercise, dietary Ca, vitamin D, other underlying diseases, etc. The incidence of pelvic fracture, which has the highest mortality, increases by 13-fold in the age group of 60 to 80 years. Osteoporosis is the most important predictor of pelvic fracture in both men and women. Therefore, efforts to reduce the incidence of osteoporosis along with prevention of falls and appropriate treatment according to BMD are needed. In addition, attention should also be paid to a variety of endocrine disruptors that can affect bone metabolism.
DEHP is a widely known endocrine disruptor that is used to soften plastic. There are 2 pathways for DEHP exposure: the oral and parenteral routes. In this paper, we used subcutaneous injection for the sustained constant blood level of DEHP and slower rise of body burden. Absorption through airborne pollutants, perfumes, cosmetics, personal hygiene products, etc., constitute DEHP exposure via the parenteral route.[12] Although DEHP contents in cosmetics and personal hygiene products are regulated, there are reports that it is still detected in many countries.[13] Various equipment made of plastics that are used in the medical industry are the cause of parenteral phthalate exposure; Luo et al.[14] have reported phthalate detection in medical products made of polyvinyl chloride. In addition, it has been reported that serum DEHP and DEHP metabolite concentrations in urine among intensive care unit patients increase by 100- to 1,000-fold.[15]
Osseous tissue is synthesized by osteoblasts and absorbed by osteoclasts. The synthesis and resorption of osseous tissue play an important role in maintaining its strength. The functions of osteoblasts and osteoclasts are affected by various hormones, among which testosterone, estrogen, and cortisol are hormones that influence the synthesis and resorption of osseous tissue. Since the cells in osseous tissues respond to estrogen, endocrine disruptors that mimic estrogen were believed to influence the functions of osteoblasts and osteoclasts; many previous studies regarding this aspect have been published.
Animal studies have shown that the oral exposure of endocrine disruptors such as phthalates to pregnant mice causes malformation and deformation in the bones of prenatal mice,[3] and affects the actin fiber of the osteoblasts, thereby transforming their shape.[16] Phthalates like benzyl butyl phthalate or di-n-butyl phthalate enter and accumulate within mice osteoblasts.[5] The structure of microfilaments in the cell is disrupted, osteoblast DNA is damaged, synthesis of apoptosis promoter proteins like P53 is promoted, and synthesis of lipid metabolism and blood is affected.[17] The differentiation of calvarial osteoblasts of mice exposed to DEHP is also affected, which is known to be due to the effects of DEHP on collagen synthesis and ALK-P expression.[18] Metabolites of phthalate like mono (2-ethylhexyl) phthalate (MEHP) or monobenzyl phthalate (MBzP) have been identified as peroxisome proliferator activated receptor γ (PPAR-γ) agonists. Increase in the PPAR-γ level further leads to a decrease in the BMD, which is known to show effects especially in postmenopausal women.[19,20]
Selective estrogen receptor modulator (SERM) and phytoestrogen are substances that affect estrogen action in the body, such as hormone disruptants. SERM drugs act on the estrogen receptor. It act as partial estrogen receptors agonists for maintaining bone density bone for applications in osteoporosis treatment, and same time act as estrogen receptor antagonists in breast tissues. Phytoestrogens are chemicals synthesized from plants, and show low estrogenic activity or anti estrogenic activity.[21] They binds to estrogen receptor and occupies it to prevent estrogen from binding to the receptor. Unlike SERM or phytoestrogen, the mechanism of action of DEHP is thought not to be through the estrogen receptor. In hepatic tissues, DEPH modulates some genetic pathways like PPAR-α signaling pathways and Janus kinase/signal transducers and activators of transcription pathway [22] and in ovarian tissues DEHP dysregulated proapoptotic factors and antiapoptotic factors and altered levels of proteins in phosphatidylinositol 3 kinase (PIsK) signaling pathways.[23,24] In a recently reported study by Chiu et al.[25], they suggested that DEHP and MEHP exposure may inhibit osteoblastogenesis and promote adipogenesis of bone marrow stromal cells in a mouse model. The downregulation of Wnt/β-catenin signaling and the upregulation of PPAR-γ pathway may contribute to the inhibitory effects of DEHP or MEHP on osteoblast differentiation and thus triggering bone loss.[25]
In human study, some authors reported about phthalate and bone health. Min and Min [11] claimed in a study with 398 women older than 50 years of age that urinary concentration of mono-n-butyl phthalate, mono-(3-carboxyprophyl) phthalate, MBzP correlates with low BMD, which increases the risk of osteoporosis in postmenopausal women. DeFlorio-Barker and Turyk [26] have demonstrated that there is a negative correlation between the total low-molecular weight phthalate metabolite contents and BMD in postmenopausal women. The relationship between phthalate metabolites and BMD is affected by body fat percentage and age; postmenopausal women younger than 65 years of age with low body fat percentage showed a negative correlation between BMD and phthalate metabolites, while women older than 65 years of age with a high body fat percentage showed a positive correlation between BMD and phthalate metabolites. The average phthalate exposure is 0.003 to 0.03 mg/kg/day (7.7-77 µM),[27] and the concentration of low dose DEHP in this paper is 30 µg/kg/day, which is relevant to the clinical situation. The dosage of high dose is over 10 times of mean exposure level of human as previously reported.[28]
The results of this study showed that in mice that were exposed to DEHP, bone formation marker levels significantly decreased, while the bone resorption marker levels significantly increased; these results differed clearly from those observed for the estrogen treatment group. In biochemical assessment, serum P level was significantly low in high dose DEHP group and serum ALK-P levels were significantly low in low dose and high dose DEHP group than control. In postmenopausal osteoporosis women, serum ALK-P is increased because of high bone turnover and serum Ca and serum P levels are decreased.[29] In other words, the effect of DEHP that act on bones is not simply due to their estrogen or anti-estrogen like function. Further studies about biochemical changes in DEHP exposed mice are needed. In addition, BMD was significantly reduced in mice treated with a high dose of DEHP, and the results of Micro-CT showed that the SMI in this group increased significantly, compared to that for other groups. SMI is the distribution of rods and plates obtained from the structural analysis of trabecular bone, which is represented as a ratio [30]; it increases with the progression of osteoporosis.[26] The increase of the SMI value indicates the onset of osteoporosis.[31,32] For a structure with both plates and rods of equal thickness the value lies between 0 and 3, epending on the volume ratio of rods and plates. In this paper, high dose DEPH group showed decreased BMD and higher SMI, which means weakened bone strength.
This study was performed under well controlled same environment to control and treatment group. In the think of many factors which could influence to bone metabolism, well controlled animal study is highly valuable. Small sample size is the limitation of this paper. In blood analysis data, we took the baseline samples, but the amount of blood was not sufficient for analysis. So we analyzed the data after 1 month. This should be taken into consideration when interpreting the results. As estrogen is a powerful protector of bone, there is likely to be a difference in the effect of DEHP on bone in situations with and without estrogen. This study was carried out in the absence of estrogen because absence of estrogen could better explain the effects of DEHP. In the further, the study with estrogen should proceed more.
The DEHP treatment groups showed lesser bone formation and greater bone resorption than the control group. In addition, Low BMD and increase of the SMI value were observed in the high-dose DEHP group, it means the progression of osteoporosis. In summary, based on the results of this study, the negative effects of DEHP on bone metabolism in ovariectomized mice were confirmed. So we could suggest that DEHP may have the possibility of adverse effect on bone metabolism in postmenopausal women. As a result of this study, it is difficult to say with certainty how DEHP affects the bones. However, it is certain that DEHP affects bone metabolism, and avoiding DEHP in menopausal women is likely to have a positive effect on bone metabolism as it gives a negative effect on high dose. Further studies on genetic pathways and other endocrine disruptors will be necessary for a deeper understanding of these effects and treatment of bone disorders.
DECLARATIONS
References
1. Roy JR, Chakraborty S, Chakraborty TR. Estrogen-like endocrine disrupting chemicals affecting puberty in humans--a review. Med Sci Monit 2009;15:Ra137-Ra145.

2. Migliaccio S, Newbold RR, Bullock BC, et al. Alterations of maternal estrogen levels during gestation affect the skeleton of female offspring. Endocrinology 1996;137:2118-2125.



3. Migliaccio S, Newbold RR, Teti A, et al. Transient estrogen exposure of female mice during early development permanently affects osteoclastogenesis in adulthood. Bone 2000;27:47-52.


4. Hermsen SA, Larsson S, Arima A, et al. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) affects bone tissue in rhesus monkeys. Toxicology 2008;253:147-152.


5. Sabbieti MG, Agas D, Santoni G, et al. Involvement of p53 in phthalate effects on mouse and rat osteoblasts. J Cell Biochem 2009;107:316-327.


6. Agas D, Sabbieti MG, Marchetti L. Endocrine disruptors and bone metabolism. Arch Toxicol 2013;87:735-751.



7. Harris CA, Henttu P, Parker MG, et al. The estrogenic activity of phthalate esters in vitro. Environ Health Perspect 1997;105:802-811.



8. Okubo T, Suzuki T, Yokoyama Y, et al. Estimation of estrogenic and anti-estrogenic activities of some phthalate diesters and monoesters by MCF-7 cell proliferation assay in vitro. Biol Pharm Bull 2003;26:1219-1224.


9. Hurst CH, Waxman DJ. Activation of PPARalpha and PPAR-gamma by environmental phthalate monoesters. Toxicol Sci 2003;74:297-308.



10. Cheon KY, Kil KH, Choi JI, et al. Parenteral exposure to DEHP and its effect on the microstructure of bone and Wnt signaling pathway in F2 female mice. Biochip J 2016;10:233-240.


11. Min KB, Min JY. Urinary phthalate metabolites and the risk of low bone mineral density and osteoporosis in older women. J Clin Endocrinol Metab 2014;99:E1997-E2003.

12. Koo HJ, Lee BM. Estimated exposure to phthalates in cosmetics and risk assessment. J Toxicol Environ Health A 2004;67:1901-1914.


13. Guo Y, Wang L, Kannan K. Phthalates and parabens in personal care products from China: concentrations and human exposure. Arch Environ Contam Toxicol 2014;66:113-119.



14. Luo H, Sun G, Shi Y, et al. Evaluation of the Di(2-ethylhexyl)phthalate released from polyvinyl chloride medical devices that contact blood. Springerplus 2014;3:58.




15. Huygh J, Clotman K, Malarvannan G, et al. Considerable exposure to the endocrine disrupting chemicals phthalates and bisphenol-A in intensive care unit (ICU) patients. Environ Int 2015;81:64-72.


16. Gordon SR. Microfilament disruption in a noncycling organized tissue, the corneal endothelium, initiates mitosis. Exp Cell Res 2002;272:127-134.


17. Agarwal DK, Maronpot RR, Lamb JCt, et al. Adverse effects of butyl benzyl phthalate on the reproductive and hematopoietic systems of male rats. Toxicology 1985;35:189-206.


18. Bhat FA, Ramajayam G, Parameswari S, et al. Di 2-ethyl hexyl phthalate affects differentiation and matrix mineralization of rat calvarial osteoblasts--in vitro. Toxicol In Vitro 2013;27:250-256.


19. Akune T, Ohba S, Kamekura S, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 2004;113:846-855.

20. Grey A, Bolland M, Gamble G, et al. The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab 2007;92:1305-1310.


21. Lecomte S, Demay F, Ferrière F, et al. Phytochemicals targeting estrogen receptors: Beneficial rather than adverse effects? Int J Mol Sci 2017;18:E1381.


22. Rusyn I, Peters JM, Cunningham ML. Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit Rev Toxicol 2006;36:459-479.



23. Hannon PR, Brannick KE, Wang W, et al. Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol Appl Pharmacol 2015;284:42-53.



24. Hannon PR, Peretz J, Flaws JA. Daily exposure to Di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol Reprod 2014;90:136.



25. Chiu CY, Sun SC, Chiang CK, et al. Plasticizer di(2-ethylhexyl) phthalate interferes with osteoblastogenesis and adipogenesis in a mouse model. J Orthop Res 2018;36:1124-1134.


26. DeFlorio-Barker SA, Turyk ME. Associations between bone mineral density and urinary phthalate metabolites among post-menopausal women: a cross-sectional study of NHANES data 2005-2010. Int J Environ Health Res 2016;26:326-345.


27. Agency for Toxic Substances and Disease Registry. Toxicological profile for di(2-Ethylhexyl)phthalate (DEHP) 2002;cited by 2017 Sep 1. Available from: https://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=684&tid=65.
28. Cho HH, Kim GW, Ryu JC. The effects of Di-2-ethylhexyl phthalates (DEHP) on the cell cycle of the endometrial cancer cell lines (ECC-1). Toxicol Environ Health Sci 2014;6:217-223.


29. Khadka B, Tiwari ML, Gautam R, et al. Correlates of biochemical markers of bone turnover among post-menopausal women. JNMA J Nepal Med Assoc 2018;56:754-758.

30. Hildebrand T, Rüegsegger P. Quantification of bone microarchitecture with the structure model index. Comput Methods Biomech Biomed Engin 1997;1:15-23.


Fig. 1
Comparison of serum bone formation marker (osteocalcin) concentrations at month 1 and 2 after di(2-ethylhexyl)phthalate (DEHP) treatment in ovariectomized mice. A gradual increase was shown in the estrogen treatment group (E), but a decrease was shown in the control group (C), low-dose DEHP group (L), and high-dose DEHP group (H). The calculated subtraction value is statistically greater in the H (*) than the C. *P<0.05 vs. C.

Fig. 2
Change in serum bone resorption marker (C-terminal telopeptide of type 1 collagen [CTX-1]) concentrations at month 1 and 2 after di(2-ethylhexyl)phthalate (DEHP) treatment. CTX-1 level showed a decrease in the estrogen treatment group (E), while an increase the other groups. The calculated subtraction value of CTX-1 is statistically greater in the low-dose DEHP group (L) (*) than the control group (C). H, high-dose di(2-ethylhexyl)phthalate treatment group. *P<0.05 vs. C.
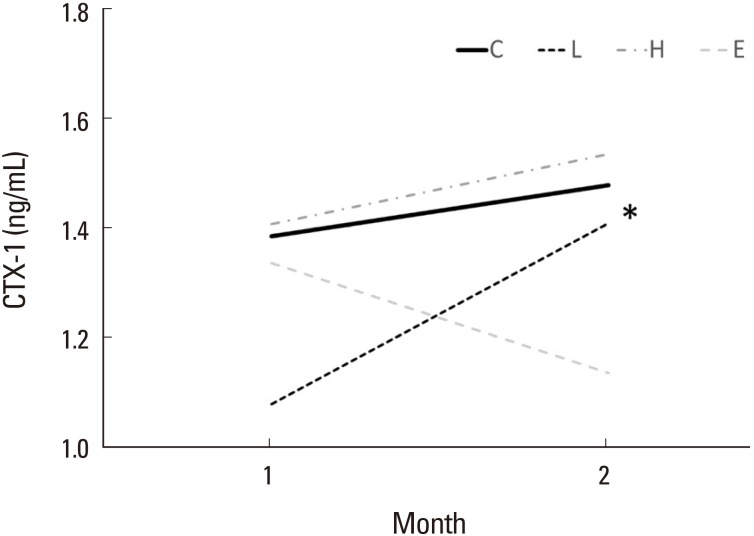
Fig. 3
Change in serum calcium (Ca), phosphorus (P), alkaline phosphatase (ALK-P), and magnesium (Mg). There was no significant difference in the serum Ca levels and Mg levels among the three groups. Although, there was a significant decrease in the serum P level in the high-dose di(2-ethylhexyl)phthalate (DEHP) group, and the serum ALK-P levels were decreased to a greater extent in the low-dose DEHP groups (L) and high-dose DEHP groups (H) than in the control group (C). *P<0.05 vs. C.

Fig. 4
Comparison of bone marrow density (BMD) in the tibia of ovariectomized mice. The BMD was significantly lower in the high-dose di(2-ethylhexyl)phthalate (DEHP) group (H) (*) and significantly higher in the estrogen-treated group (E) than control group (C). L, low-dose d di(2-ethylhexyl)phthalate treatment group. *P<0.05 vs. C.
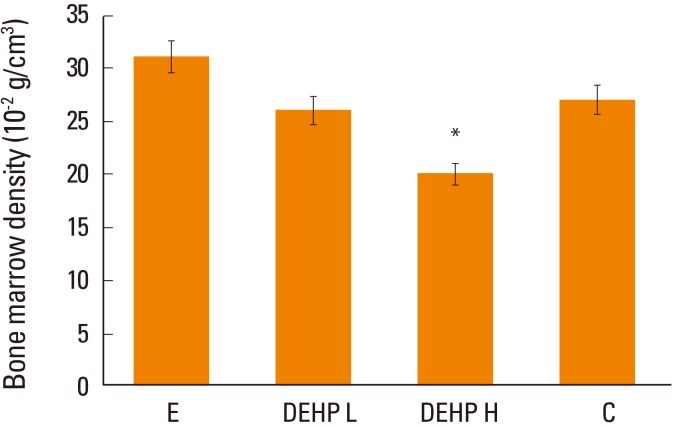
Fig. 5
Tibia head microstructure analysis using micro-computed tomography scan. Trabecular area (yellow) is more prominent in estrogen treatment group (E). In high-dose di(2-ethylhexyl)phthalate (DEHP) group (H), trabecular area is smaller than the others (E, control group [C], low-dose DEHP treatment group [L]).

Fig. 6
Structure model index (SMI) analysis in the tibia of ovariectomized mice. SMI was significantly higher in the high-dose di(2-ethylhexyl) phthalate (DEHP) group (H) (*) than in the estrogen treatment (E) and low-dose DEHP groups (L). No significant differences were observed in the H and control group (C). *P<0.05 vs. C.
- TOOLS
-
METRICS

- Related articles
-
Effect of Dexamethasone on Bone Resorption in Organ-cultured Fetal Rat Bone1998 November;5(2)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print