Anorexia Nervosa and Osteoporosis: Pathophysiology and Treatment
Article information
Abstract
Anorexia nervosa (AN) affects 2.9 million people, many of whom experience bone loss and increased fracture risk. In this article, we review data on the underlying pathophysiology of AN-related osteoporosis and possible approaches to disease management. Available research suggests that low body weight and decreased gonadal function are the strongest predictors of bone loss and fractures in patients with AN. Additionally, other metabolic disturbances have been linked to bone loss, including growth hormone resistance, low leptin concentrations, and hypercortisolemia, but those correlations are less consistent and lack evidence of causality. In terms of treatment of AN-related bone disease, weight gain has the most robust impact on bone mineral density (BMD). Restoration of gonadal function seems to augment this effect and may independently improve BMD. Bisphosphonates, insulin-like growth factor 1 supplementation, and teriparatide may also be reasonable considerations, however need long-term efficacy and safety data.
INTRODUCTION
Anorexia nervosa (AN) is characterized by intense fear of weight gain resulting in calorie restriction, weight loss, and pathologically low body weight. It has a prevalence of 2.9 million people worldwide [1] with a yearly incidence of 8 per 100,000.[2]
AN is associated with a 3-fold increase in the lifetime risk of fractures [3] with up to 57% of women with AN sustaining at least 1 fracture in their lifetime.[4] Much of this risk is due to reduced bone mineral density (BMD); 38% of patients with AN have T scores <−2.5 and 92% have T scores <−1.[5] This BMD reduction is believed to be caused by varying degrees of increased bone resorption and decreased bone formation. Observational studies have shown that bone formation markers such as osteocalcin and bone-specific alkaline phosphatase (BSAP) are decreased in adult patients with AN and low BMD, while bone resorption markers such as C-terminal telopeptide of type I collagen (CTX) and N-terminal telopeptide of collagen type I are elevated.[6789]
Unfortunately, there is limited research that clearly elucidates the specific causes and the most effective approaches to treatment of bone disease in patients with AN. In this article, we provide an updated summary of existing data regarding the pathophysiology and treatment of AN-related osteoporosis.
METHODS
A manual search was conducted in PubMed, Medline, Cochrane Library, and ClinicalTrials. Studies were encountered using search terms AN, osteoporosis, osteopenia, BMD, bone density, weight gain, menstrual regulation/amenorrhea/hypogonadism, fractures, hormones (leptin, ghrelin, thyroxine, oxytocin, cortisol, estrogen, testosterone, insulin-like growth factor 1 [IGF-1]) and treatments (risedronate, alendronate, menatetrenone, teriparatide, denosumab). Wildcard qualifier (*) was affixed to each search term. Only studies that specifically evaluated AN and bone health were included.
WHAT FACTORS CONTRIBUTE TO AN-RELATED BONE DISEASE?
In AN, the body is in a state of starvation and low energy leading to numerous metabolic and physiologic alterations that are described individually in this section.
1. Amenorrhea and gonadal hormones
Alterations of menstrual cycles are common, seen in up to 70% of females with AN.[4] This typically manifests with amenorrhea, decreased estrogen concentrations, and low or low-normal gonadotropins suggesting central suppression of the hypothalamic-pituitary-gonadal axis.[10] Low BMD is often seen in amenorrheic women, while eumenorrhea seems to be protective against bone loss.[11] Estrogen deficiency is believed to be the main factor mediating AN-related bone loss [12] and several studies have shown a direct correlation between estradiol concentrations and BMD in patients with AN.[111314] This is likely due to the loss of the inhibitory effect of estrogen on osteoclasts leading to increased bone resorption.[1516] Notably, women with AN who remain eumenorrheic have estradiol concentrations about 3 times higher compared to amenorrhoeic patients with similar body mass index (BMI) [17] and better BMD in this subgroup of patients has been attributed to their higher estradiol concentrations.
Longer duration and earlier age of onset of amenorrhea both correlate with lower BMD.[8121819] Biller et al.[12] demonstrated that women with AN and peripubertal onset of amenorrhea have a 20% larger BMD deficit compared to those who experience amenorrhea after completion of the pubertal transition. Since puberty is a time of increased bone formation, it stands to reason that alterations in hormones that affect bone accrual during this period of time (e.g. decreased estrogen) are likely to result in more deleterious effects on BMD,[20] as achievement of peak bone mass during adolescence and early adulthood may attenuate the risk of osteoporosis later in life.
Women with AN and low BMD also have lower concentrations of testosterone [6] and dehydroepiandrosterone (DHEA).[21] However, since testosterone naturally undergoes aromatization to estrogen,[16] it is difficult to elucidate whether the low BMD is due to the low testosterone itself or to decreased estrogen activity.
Males with AN have been noted to have lower testosterone concentrations which correlate with lower BMD,[22] but no studies have assessed the effect of androgen replacement on BMD in these patients. In hypogonadal men without AN, testosterone therapy improves BMD,[23] but administration of synthetic androgens which cannot be converted to estrogens has no effect on BMD.[23] This again suggests that testosterone exerts much of its effect on bone through its aromatization to estrogen.
2. Body mass index and leptin
By definition, patients with AN have low BMI.[24] Lower BMI correlates with lower BMD [2526] and the lowest lifetime BMI in these patients predicts a higher risk of osteoporosis.[27282930] In addition, the duration of time of low BMI inversely correlates with their BMD.[58]
Leptin is a hormone directly affected by weight. It is secreted by adipocytes when energy stores are increased, but its production is decreased in low energy states such as AN [31] due to low fat mass. In patients with AN, a direct correlation exists between leptin concentrations and BMD, independent of BMI.[1327]
3. Other hormonal alterations
Research by Misra et al.[32] showed that patients with AN manifest elevated growth hormone (GH) and low IGF-1 concentrations, suggesting some degree of resistance to GH. Since IGF-1 exerts stimulatory effects on osteoblasts,[33] it has been postulated that the low IGF-1 in patients with AN may contribute to their low bone density.[2834] Later studies have confirmed an association between IGF-1 and BMD in patients with AN independent of their BMI.[1335]
Cortisol, a catabolic hormone, is increased in AN likely as a reflection of chronic physiologic stress.[36] In general, hypercortisolemia is deleterious to bone by inhibiting osteoblast proliferation and bone formation [36] and studies in patients with AN suggest an inverse relation between cortisol concentrations and bone density.[2737] It is however unclear whether this observed correlation reflects a direct effect of cortisol on bone or if the hypercortisolemia is simply a physiologic reflection of the severity of the underlying eating disorder.
Lastly, oxytocin has been shown to induce osteoblast formation and to inhibit osteoclast activity in mice.[38] Oxytocin concentrations are decreased in patients with AN,[39] but return to normal with weight gain.[40] Decreased oxytocin concentrations in these patients predict lower BMD even after correction for BMI [41] but no studies have evaluated the effect of oxytocin administration on bone health in AN.
4. Role of exercise
A tendency toward excessive exercise is seen in 31% to 80% of patients with AN [42] and typically presents with a compulsion to exercise regardless of weight or physical state. While exercise is beneficial to bone health in the general population,[43] it might be deleterious in the case of a patient with active AN whose body is already in a low energy state.[42] A recent study by Waugh et al.[44] showed that exercising while ill from AN decreases BMD, while high bone loading activities when AN is in remission (defined as BMI >18 and recovery of menstrual cycles) leads to an increase in BMD, suggesting exercise is beneficial when appropriately timed.
CAN AN-RELATED BONE DISEASE BE STABILIZED OR REVERSED?
Based on the physiologic and hormonal alterations seen in patients with AN described in the previous section, several therapeutic approaches to improve bone health in these patients have been studied and will be described here.
1. Weight restoration
Weight gain is generally considered the most effective intervention to attenuate or reverse bone loss in patients with AN. Several studies have assessed the effect of weight restoration on BMD as summarized in Table 1 [193045464748495051525354555657] and many showed positive correlations between weight gain and BMD. In a secondary analysis of patients randomized to alendronate or placebo, Golden et al.[51] compared changes in BMD between patients who experienced weight gain during the study to those who did not. Weight restoration (defined as a weight at or above 85% of standard body weight) was associated with an increase in BMD at the hip and spine that was independent of both alendronate administration and resumption of menses. Another study by Miller et al.[53] showed that weight gain, independent of oral contraceptive use or regulation of menstrual cycles, improved BMD at the hip.
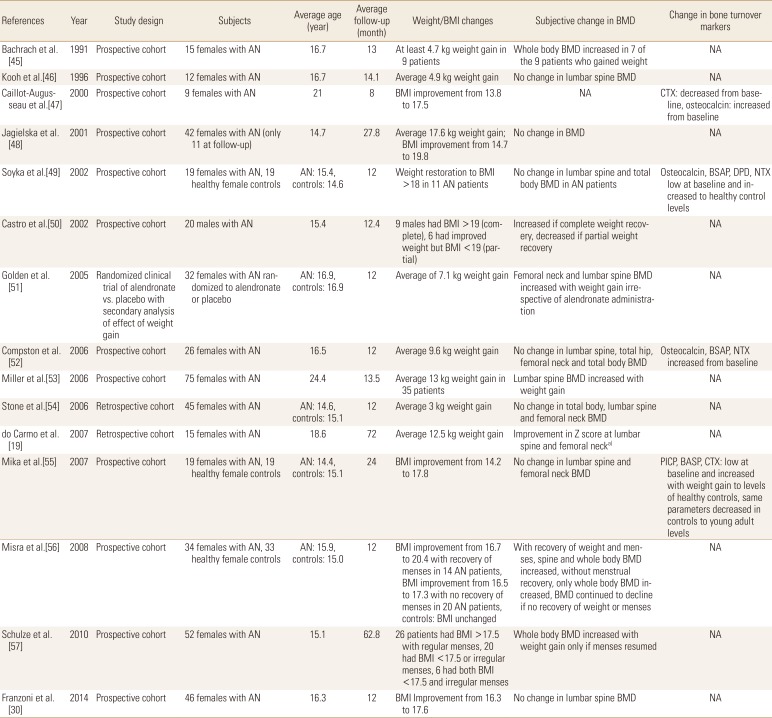
Summary of studies showing effect of weight gain on bone mineral density and serological markers in patients with anorexia nervosa
Most of these studies unfortunately did not provide a detailed nutritional regimen or protocol to induce sufficient weight gain in these patients. Only 3 studies provided rather limited information regarding their refeeding protocols.[525455] These generally included high dietary protein,[545558] graduated increases in daily caloric intake to a target of 2,500 to 4,000 kcal/day,[525558] and a daily calcium intake of around 1,500 to 2,000 mg.[545558]
It is important to note here that while several other studies failed to demonstrate an increase in BMD in association with weight restoration, none showed loss of BMD in association with weight gain.[30464849525455] In the setting of AN where continued weight loss and malnutrition might result in continued bone loss, stabilization of BMD as a result of weight restoration may actually present a favorable outcome. Furthermore, many of these studies did show favorable changes in markers of bone turnover, perhaps suggesting early improvements in bone metabolism not yet captured by BMD measurement.[47495255] For example, Compston et al.[52] evaluated 21 young females who experienced an average of 10 kg of weight gain over a period of 1 year and did not observe improvements in their BMD. However, they noted an increase in the concentrations of markers of bone formation and a decrease in the concentrations of markers of bone resorption, suggesting a positive effect of weight gain that likely had not yet manifested as a change in BMD.[52]
It is important to note here that some of the positive studies showed improvement in BMD as a function of weight gain alone,[45505153] while others suggested that both weight restoration and resumption of menses were essential for the BMD to increase.[5657] Since traditional estrogen replacement therapy with oral contraceptive pills (OCPs) in patients with active AN does not seem to be consistently effective at improving BMD (as will be discussed in the next section), it is fair to assume that weight restoration, with or without correction of hypogonadism, is an essential intervention to stop or reverse bone loss.
SEX HORMONE REPLACEMENT
1. Estrogen
Early observational studies suggested that OCP use [59] or use of estrogen replacement [60] was associated with improvement in BMD in adults with AN. However, prospective clinical trials using typical OCP doses (35–50 µg of oral estradiol) for 1 and 2 years did not have any effect on BMD in peripubertal AN patients.[53616263] Conversely, administration of estradiol at more physiologic doses to peripubertal females with AN (100 µg transdermally in patients with bone age >15 years, or escalating doses of 3.75–11.25 µg oral in those with bone age <15 years) led to near-normalization of BMD after 18 months of therapy.[64] It is unclear whether this robust change was due to the route of administration of estrogen which bypassed hepatic metabolism or the more physiologic doses used. Further studies are needed to clarify this question.
2. Androgens
When 33 women with AN and low testosterone concentrations were treated with transdermal testosterone (150 or 300 µg daily patches) for 3 weeks, they experienced an increase in CTX with no changes in osteocalcin and BSAP.[65] A later study randomized 19 women to testosterone alone (150–300 µg daily transdermal patch based on serum testosterone concentrations), 20 to risedronate alone (35 mg/week), 20 to dual therapy with testosterone and risedronate, and 18 to placebo. After a 12-month follow-up, no improvements in bone turnover markers or BMD were observed in the testosterone monotherapy or placebo groups. Only patients who took risedronate (either alone or in combination with testosterone) experienced an improvement in BMD. However, there was no difference in the observed benefit between patients who received risedronate alone and those who received testosterone and risedronate,[66] suggesting that testosterone supplementation is unlikely to be of benefit in the treatment of osteoporosis in women with AN.
Treatment of women with AN with DHEA at doses ranging between 50 and 100 mg daily does not increase BMD compared to baseline or to untreated controls according to 2 randomized clinical trials.[6768] When DHEA was given in combination with an OCP (DHEA 50 mg+20 µg ethinyl estradiol/0.1 mg levonorgestrel) for 18 months, BMD remained stable while untreated controls experienced a drop in BMD,[6970] suggesting that DHEA and OCPs may attenuate bone loss when used in combination, though there are still very limited data supporting this notion.
ANTI-RESORPTIVE AGENTS
1. Bisphosphonates
Two oral bisphosphonates, risedronate and alendronate, have been studied in AN. In a randomized clinical trial of 32 adolescent girls with AN who took alendronate 10 mg daily or placebo for 1 year, the end-of-study BMD was significantly higher than baseline in the alendronate group but not in the placebo group. However, the magnitude of change in BMD from baseline between the 2 groups was not statistically different (4.4% vs. 2.3% at the femoral neck and 3.5% vs. 2.2% at the lumbar spine with alendronate and placebo respectively).[51]
In a small study of 10 women with AN and low BMD, risedronate 5 mg daily increased BMD at the spine by 4.1% and 4.9% at 6 and 9 months, respectively.[71] A larger study where 38 women were randomized to risedronate 35 mg weekly or placebo showed a 3.2% increase in lumbar spine BMD in the risedronate group after 12 months of treatment compared to no change in the control group. This study also assessed the effect of testosterone supplementation on bone density (both alone and in combination with risedronate as described in a previous section) and showed no improvement with testosterone.[66]
Based on these data, it appears that there may be a trend toward improved BMD with bisphosphonates, but the evidence is still insufficient at this point.
2. Denosumab
In a case report, denosumab use for 3 years (at 60 mg subcutaneously every 6 months) in a woman with AN and low BMD led to a 14.8% increase in BMD at the spine, a 1.4% increase at the hip, and a 5.7% increase at the femoral neck.[72] In a separate report, treatment with denosumab (same dosing) in 3 women was again associated with an improvement in BMD at the hip by 20% and at the lumbar spine by 17%.[73] The role of denosumab in the treatment of AN-related osteoporosis has not been formally evaluated, and further studies are necessary to clarify these anecdotal findings.
ANABOLIC AGENTS
1. Teriparatide
In 2012, a case report of teriparatide treatment of a postmenopausal woman with AN and osteoporosis showed improvement in BMD by 12% at the lumbar spine and 21% at the femoral neck when administered in conjunction with vitamin D supplementation and weight gain.[74] Later, a randomized controlled trial of 21 women treated with teriparatide (20 µg subcutaneously daily) or placebo showed that teriparatide increased BMD at the lumbar spine by 6% within 6 months,[75] even after correcting for baseline body weight.
2. IGF-1/Growth hormone
As IGF-1 concentrations are low in patients with AN, several studies have assessed the effect of IGF-1 replacement on BMD. In fact, women with AN and low BMD manifested a dose dependent increase in markers of bone formation when given recombinant human IGF-1 at either 60 or 200 mcg subcutaneously daily.[7] In another study, administration of recombinant human IGF-1 (60–80 µg daily) to adolescents with AN increased markers of bone formation and bone resorption.[76] Grinspoon et al.[77] randomized 60 osteopenic women with AN to four groups for 12 weeks: IGF-1 monotherapy (30 µg/kg twice daily), OCP monotherapy (35 µg ethinyl estradiol/0.4 mg norethindrone daily), combination therapy with OCP and IGF-1 at the same doses, or placebo. The patients in the IGF-1 monotherapy and the IGF-1/OCP groups experienced an increase in bone density at the lumbar spine of 1.1% and 1.8%, respectively, while BMD was unchanged in the other 2 groups. The increase in BMD from baseline in each of the IGF-1 and IGF-1/OCP groups were significant, but there was no difference in the magnitude of the change between the 2 groups. This suggests that IGF-1 may be modestly effective at increasing BMD, but that OCPs may not augment the efficacy of IGF-1.[77] However, it is important to note that the short duration of the study may have been insufficient to fully demonstrate changes in BMD.
Interestingly, in 21 adults with AN, treatment with recombinant GH (dose was titrated to achieve IGF-1 levels in the upper quartile of the normal range) did not affect markers of bone turnover. These patients required GH doses 3 times higher than doses used in patients with GH deficiency due to pituitary disease (15 vs. 5 µg/kg), which further supports the theory of GH resistance in AN.[78]
OTHER THERAPIES
1. Leptin
The correlation between higher leptin concentrations and increased BMD makes leptin a promising therapeutic target, but it has not been evaluated in patients with AN. In patients with exercise-induced hypothalamic amenorrhea, its administration lead to an increase in markers of bone formation (BSAP and osteocalcin) in one study [79] and to a 4% to 6% improvement in lumbar spine BMD in another.[80] However, patients who took leptin experienced a small degree of weight loss,[7980] an effect that might preclude its safe use in patients with AN given the baseline weight deficits in these patients.
2. Menatetrenone
Vitamin K stimulates osteoblast activity through vitamin K dependent carboxylation of osteocalcin.[81] Menatetrenone is a vitamin K analogue that works similarly to stimulate this pathway. A single study in 19 young women with AN showed that treatment with menatetrenone for 1 year attenuated BMD loss by 4% (2.9% BMD loss in treatment arm versus 6.9% BMD loss in control arm).[82] Further research with longer follow-up will be needed to better understand the role of menatetrenone on bone health in patients with AN.
DISCUSSION
Our review of the literature suggests that low body weight and decreased gonadal function are the strongest predictors of bone loss and fractures in patients with AN. While other metabolic disturbances such as GH resistance, low leptin concentrations, and hypercortisolemia have been linked to bone loss, those correlations are less consistent and lack evidence of causality.
In terms of treatment of AN-related bone disease, weight gain has the most robust impact on BMD. In addition, restoration of gonadal function seems to augment this effect and may independently improve BMD. Bisphosphonates, IGF-1 supplementation, and teriparatide may also be reasonable considerations, but still need long-term efficacy and safety data. Most notable here is the inconsistency in efficacy between risedronate and alendronate, despite the fact these drugs have fairly similar mechanisms of action.[8384] Whether this discrepancy reflects a true difference in clinical efficacy versus variability in study design is unclear, highlighting the fact that there is no sufficient evidence at this time to support the universal use of bisphosphonates in AN-related bone disease.
Limitations of the studies reviewed include the rather small size and short duration of follow up of most studies and the significant heterogeneities in relation to subjects (some studies were focused on either adolescents or adults, while others included both age groups) and interventions (for example, studies on OCPs used variable doses of estrogen). Furthermore, in 2013, the Diagnostic and Statistical Manual of Mental Disorders, fourth edition was published and changed the definition of AN to no longer require amenorrhea for diagnosis,[24] which is especially critical in osteoporosis as menstrual status can affect bone health.
It is also important to note that all studies used surrogates of bone health as outcomes, namely BMD and bone turnover markers, and that no studies looked at the effect of interventions on fracture risk. While improvements in these surrogate markers are generally assumed to imply decreased fracture risk, direct correlation of interventions with fracture risk will ultimately be essential to truly prove efficacy.
Notes
Ethics approval and consent to participate: Not applicable.
Conflict of interest: No potential conflict of interest relevant to this article was reported.
