The Effect of Antidepressants on Mesenchymal Stem Cell Differentiation
Article information
Abstract
Background
Use of antidepressant medications has been linked to detrimental impacts on bone mineral density and osteoporosis; however, the cellular basis behind these observations remains poorly understood. The effect does not appear to be homogeneous across the whole class of drugs and may be linked to affinity for the serotonin transporter system. In this study, we hypothesized that antidepressants have a class- and dose-dependent effect on mesenchymal stem cell (MSC) differentiation, which may affect bone metabolism.
Methods
Human MSCs (hMSCs) were committed to differentiate when either adipogenic or osteogenic media was added, supplemented with five increasing concentrations of amitriptyline (0.001–10 µM), venlafaxine (0.01–25 µM), or fluoxetine (0.001–10 µM). Alizarin red staining (mineralization), alkaline phosphatase (osteoblastogenesis), and oil red O (adipogenesis) assays were performed at timed intervals. In addition, cell viability was assessed using a MTT.
Results
We found that fluoxetine had a significant inhibitory effect on mineralization. Furthermore, adipogenic differentiation of hMSC was affected by the addition of amitriptyline, venlafaxine, and fluoxetine to the media. Finally, none of the tested medications significantly affected cell survival.
Conclusions
This study showed a divergent effect of three antidepressants on hMSC differentiation, which appears to be independent of class and dose. As fluoxetine and amitriptyline, but not venlafaxine, affected both osteoblastogenesis and adipogenesis, this inhibitory effect could be associated to the high affinity of fluoxetine to the serotonin transporter system.
INTRODUCTION
Maintenance of the human skeleton is dependent on the balance between bone deposition and bone resorption, which are mediated by osteoblasts and osteoclasts, respectively.[1] Failure to maintain bone mass can lead to an architectural decline in bone structure, which results in osteoporosis and a predisposition to fractures.[1] Mesenchymal stem cells (MSC) within the bone marrow differentiate into osteoblasts and thus play an important role in bone integrity.[2] There is an increase in volume of adipose tissue and a decrease in bone formation in osteoporotic bone,[3] suggesting an inverse relationship between imbalanced adipogenesis and osteoblastogenesis, and the lipotoxic effect of marrow adipocytes on other bone cells.[45]
MSC are multipotent, non-hematopoietic, self-renewing cells that have the capability to differentiate into various mesenchymal cell types including adipocytes, osteoblasts, chondrocytes, myocytes, and neurons.[678] Molecular factors, including bone morphogenic proteins, Wnt proteins and several transcription factors, are responsible for the mechanism of MSC commitment and differentiation into either osteoblasts or adipocytes.[910] Alterations in this differentiation process, such as those observed in osteoporosis, facilitate adipogenesis and affect bone formation.[5]
Derived from the amino acid tryptophan,[11] serotonin is a crucial neurotransmitter that has been associated with sleep/wake cycles,[12] cognition,[13] and memory [14] in the central nervous system (CNS). The major source of serotonin in the CNS is serotonergic neurons in the raphe nuclei located in the brainstem, which project to several important areas in the brain.[151617] However, most serotonin is produced outside the CNS, particularly in enterochromaffin cells of the gut, but also in lung endothelium and platelets, and is responsible for gastrointestinal function and vasoconstriction.[1819] Serotonin in the CNS to suppresses appetite, while peripheral serotonin appears to stimulate adipose deposition.[2021] In addition, serotonin has an inhibitory effect on osteoblastogenesis in vitro.[19] Interestingly, gut-derived serotonin (GDS) has also been shown to regulate osteoblastogenesis and bone formation in vivo.[1922] In contrast, the effect of serotonin (central or GDS) on adipogenic differentiation of MSC remains unexplored.
Tricyclic antidepressants were among the initial drug classes available to treat depression. Amitriptyline is a tricyclic antidepressant that acts primarily as an define as inhibitor constant of serotonin and noradrenaline transporters with a Ki of 4.3 nM and 35 nM respectively,[23] but also has affinity for the dopamine transporter, as well as serotonin, dopamine, adrenergic and histamine receptors. These drugs act primarily by blocking serotonin and noradrenaline transporters, thus preventing reuptake of these neurotransmitters and extending their time within the synaptic cleft.[24] However, these drugs also target additional receptors including α-adrenergic, muscarinic cholincholinergic and histamine receptors.[24] Modulation of these other receptors can manifest in undesirable side effects such as dry mouth and constipation, and for this reason, tricyclic compounds are no longer used as a medication in most cases of depression.
Currently, the drug class of choice for most cases of depression and anxiety requiring treatment are the selective serotonin reuptake inhibitors (SSRIs).[2526] While selectively targeting the serotonin transporter (5-HTT) with considerably less affinity for other receptors, these drugs have fewer side effects than tricyclic antidepressants and are generally better tolerated.[27] In terms of chemical structure and specific targets, fluoxetine is an SSRI with specific inhibition constants (Ki concentration of 1 nM and 10 µM against the 5-HTT and other serotonin receptors respectively [282930]). Venlafaxine is a dual serotonin-noradrenaline reuptake inhibitor with a Ki of 82 nM for 5-HTT and 2,480 nM for the noradrenaline transporter.[31]
Previous studies have shown a link between bone mineral density (BMD) and depression or antidepressant use. Clinical data have suggested an association between a low BMD, increased tendency to fracture, and symptoms of depression.[3233343536] In addition, low BMD and/or increased tendency to fracture appears to correlate with antidepressant use, particularly among specific antidepressant drug classes.[37]
Intrigued by these clinical data showing an association between antidepressant use and bone architecture, we sought to determine whether there is an interaction between these drugs and MSC, and whether this would affect their differentiation – including adipogenesis. In this study, we hypothesized that the presence of antidepressants would affect both differentiation and survival of MSC. Furthermore, we hypothesized that each class of antidepressant would have a varying influence on MSC differentiation.
METHODS
1. Cell cultures
Human MSC (hMSC) were obtained commercially from Lonza (Basel, Switzerland), and cultured as previously described.[3839] Briefly, cells were plated in six-well culture dishes containing MSC growth media (MSCGM; Lonza), and grown at 37℃ in a humidified atmosphere containing 5% CO2 with media changes every 3 to 4 days.
At 60% confluence, cells were harvested by trypsinization, re-plated in 96-well plates, and induced to differentiate to either osteoblasts or adipocytes. Osteoblastogenesis was achieved by replacing MSCGM with osteoblastogenesis induction media (OIM) consisting of Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 0.1 mM dexamethasone, 10 mM β-glycerophosphate, 0.05 mM ascorbic acid and penicillin/streptomycin/amphotericin B. Cell cultures were maintained for 3 weeks in OIM with media changes every 3 to 4 days prior to evaluation of differentiation characteristics.
Adipogenesis was achieved by replacing MSCGM with adipogenic induction media (AIM) consisting of DMEM supplemented with 10% FBS, 1.0 µM dexamethasone, 10 µg/mL insulin, 0.2 mM indomethacin, 0.5 mM 3-isobutyl-1-methylxanthine, and penicillin/streptomycin/amphotericin B.After 3 to 4 days, media were changed to adipogenesis maintenance media (AMM) consisting of DMEM supplemented with 10% FBS, 10 µg/mL insulin, and penicillin/streptomycin/amphotericin B. Media were changed every 3 to 4 days alternating between AIM and AMM for two weeks.
2. Alkaline phosphatase assay
To determine the extent of osteoblastogenesis, cells were assayed for alkaline phosphatase activity. Differentiated cells were drained of culture media and washed with Tris-buffered saline. Cells were lysed in lysis buffer (50 mM sodium bicarbonate, 1 mM magnesium chloride, 0.1% Triton X-100, pH 9.6) for 10 min. Reaction buffer (50 mM sodium bicarbonate, 1 mM magnesium chloride, 9 mM para-nitrophenyl phosphate (Sigma-Aldrich, St. Louis, MO, USA), pH 9.6 was added to the wells and incubated for 30 to 60 min. The colorimetric product produced (para-nitrophenol) is proportional to the amount of alkaline phosphatase and was quantified by measuring absorbance at 405 nm on a FLUOstar Optima II plate reader.
3. Mineralization assay
To assess mineralization, undifferentiated hMSC were plated in 24-well plates and differentiated with OIM as described above. Cells were fixed to the culture plates using neutral formalin and rinsed with acidic phosphate buffered saline. This was followed by staining with 2% Alizarin red (AR) solution at pH 4.2 for 10 min. Cells were rinsed then solubilized with 10% cetylpyridinium chloride in 10 mM sodium phosphate at pH 7.0 for 15 min and absorbance values were taken at 570 nm. As controls for all experiments, wells containing undifferentiated hMSC were treated with reagents, and wells containing no cells (reagents only) were also evaluated.
4. Adipogenesis assay
To determine the extent of adipogenesis and production of intracellular lipid, cells were seeded in 24-well plates and allowed to differentiate. After two weeks of differentiation as described above, cells were fixed with neutral formalin and washed with 60% isopropanol. Cells were then stained with 3% ORO (Sigma-Aldrich) in 60% isopropanol for 10 min. Cells were counterstained with hematoxylin. Representative micrographs were taken. Cells were rinsed then solubilized with 100% isopropanol for 10 min and absorbance values were determined at 500 nm. As controls, wells containing undifferentiated hMSC were stained and wells containing no cells (reagents only) were also evaluated.
5. Cell viability
To assess the viability of cells during the experiments, which could influence the resulting data, parallel experiments were run under identical conditions during the growth and differentiation phases. After osteoblastogenesis or adipogenesis, cells were treated with 0.5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT; Sigma-Aldrich) reagent for 2 to 4 hr at 37℃. Cells were then lysed with lysis buffer (0.1 M HCl, 10% Triton X-100, dissolved in isopropanol), and absorbances were taken at 490 nm on a microplate spectrophotometer. Greater absorbance values correlate to greater viability. Absorbances were subtracted from background readings (reagents and media alone in empty wells). The viability of drug-treated, differentiated cells was compared with vehicle-treated, differentiated cells, and undifferentiated hMSC.
6. Drug treatments
Fluoxetine (N-methyl-3-[(4-trifluoromethyl) phenoxy]-3-phenylpropylamine hydrochloride), venlafaxine (1-[2-(dimethylamino)-1-(4-methoxyphenyl) ethyl] cyclohexanol hydrochloride) and amitriptyline (3-(10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5-ylidene)-N,N-dimethyl-1-propanamine hydrochloride) (Sigma-Aldrich) were prepared in ultrapure water to a concentration of 10 mM. Further dilutions were made in appropriate culture media as described in the text. These concentrations are similar to therapeutic serum concentrations of these drugs in humans. Treatment with drugs (or vehicle control [VEH]) was commenced at the beginning of each differentiation processes and maintained through all subsequent media changes. Representative light micrographs (magnification×10) were taken at week two and three of differentiation.
7. Data and statistical analysis
Statistical analyses were performed with GraphPad Prism software using one-way analysis of variance with Dunnett's post-hoc test. The statistical level of significance was set at 0.05.
RESULTS
1. Effects of antidepressants on mineralization
To determine whether, as suggested by clinical data, co-incubation with antidepressants affects mineralization in vitro, we perform AR S staining at different timed intervals. Co-incubation with amitriptyline showed a modest but significant decrease in mineralization only at the highest concentration tested, 10 µM (P<0.05) (Fig. 1A, B). Whereas co-incubation with venlafaxine showed no significant change in mineralization (Fig. 1C, D), the addition of fluoxetine decreased mineralization at all concentrations tested (P<0.05) (Fig. 1E, F).
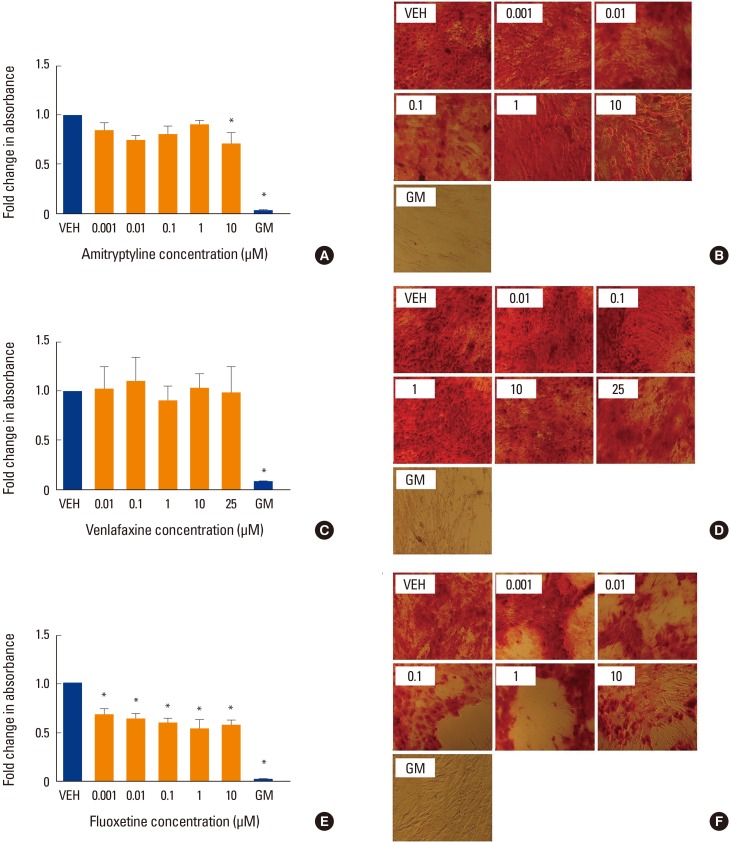
Effect of antidepressants on mineralization. Human mesenchymal stem cells (MSCs) were induced to differentiate into osteoblasts for 3 weeks at 37℃ in the presence of various concentrations of (A, B) amitriptyline, (C, D) venlafaxine, or (E, F) fluoxetine. Differentiating cells were also incubated with vehicle (VEH) alone, while undifferentiated cells were incubated with MSC growth media (GM) for 3 weeks as controls. Cells were then fixed and stained with Alizarin red (AR) as described in the methods. Changes in absorbance were measured relative to VEH only control. The extent of AR staining was quantified by solubilizing the stain with cetylpyridinium chloride and measuring the absorbance. Ratios were obtained by comparing absorbances with that of VEH. (A, B) Co-incubation with amitriptyline showed a modest decrease in AR staining only at the highest concentration tested, 10 µM. (C, D) Whereas co-incubation with venlafaxine showed no significant change in staining, (E, F) the addition of fluoxetine decreased staining at all concentrations tested. Undifferentiated hMSC showed no AR staining (GM bars). Data are representative of 3 to 6 independent experiments per drug. Images were taken under ×10 magnification. *P<0.05 compared to VEH-treated cells.
2. Effects of antidepressants on osteoblast differentiation
The level of alkaline phosphatase activity is an alternative indicator of osteogenesis with increasing activity related to increasing osteoblast phenotype. When differentiated to osteoblasts, hMSC showed approximately 2-fold increase in alkaline phosphatase activity in the absence of drug treatments. However, none of the three drug treatments caused a significant change in alkaline phosphatase activity compared to VEH control (Fig. 2).
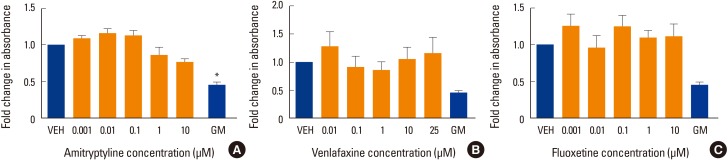
Effect of antidepressants on alkaline phosphatase activity. Alkaline phosphatase activity was measured in human mesenchymal stem cells (MSCs) that had been induced to differentiate into osteoblasts over 3 weeks at 37℃ in the presence of various concentrations of (A) amitriptyline, (B) venlafaxine, or (C) fluoxetine. Differentiating cells were also incubated with vehicle (VEH) alone, while undifferentiated cells were incubated with MSC growth media (GM) for 3 weeks as controls. Alkaline phosphatase activity is displayed relative to VEH control. Data are representative of 3 to 6 independent experiments per drug. *P<0.05 compared to VEH-treated cells.
3. Effects of antidepressants on adipogenesis
There are little data in the literature concerning the effects of antidepressants on adipogenesis in hMSC. To further investigate, we differentiated hMSC in the presence of amitriptyline, venlafaxine or fluoxetine under adipogenic conditions. When differentiated under these conditions in the absence of antidepressant medications, hMSC demonstrated an increase in lipid accumulation (lipid droplets). All three drugs tested showed a decrease in lipid staining (P<0.05) (Fig. 3A-F), with 10 µM fluoxetine showing the greatest decrease in staining (P<0.05) (Fig. 3E, F).
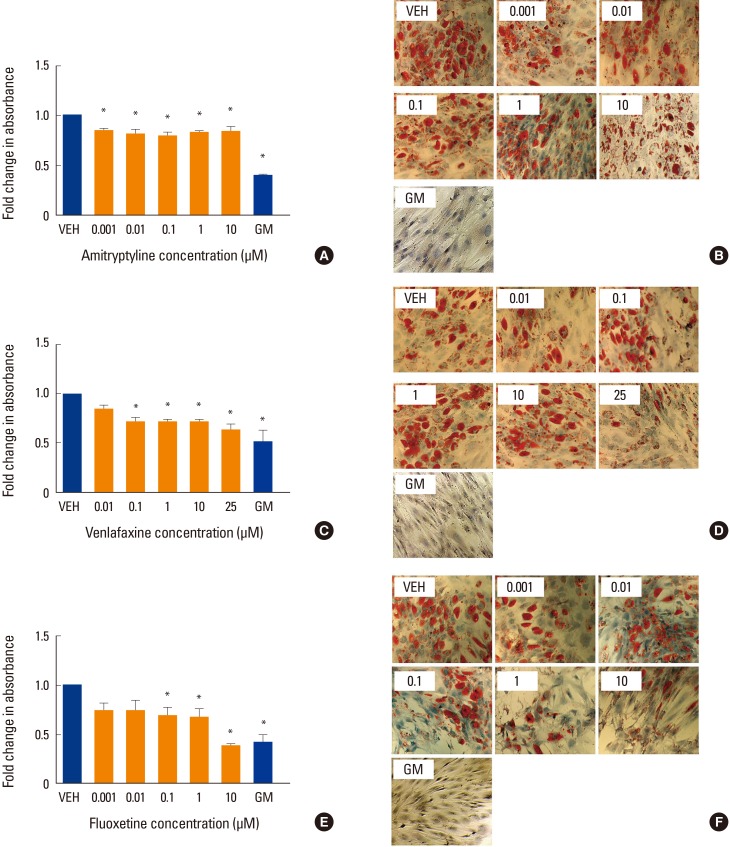
Effect of antidepressants on adipogenesis. Human mesenchymal stem cells were induced to differentiate into adipocytes for 2 weeks at 37℃ in the presence of various concentrations of (A, B) amitriptyline, (C, D) venlafaxine, or (E, F) fluoxetine. Differentiating cells were also incubated with vehicle (VEH), while undifferentiated cells were incubated with MSC growth media (GM) for 2 weeks as controls. Cells were then fixed, stained with oil red O (ORO) and counterstained with hematoxylin as described in the methods. The extent of staining was then quantified by solubilizing the stain as described in the methods. Intensity of staining was determined by absorbance relative to VEH. Data are representative of 3 to 6 independent experiments per drug. Images were taken under ×10 magnification. *P<0.05 compared to VEH-treated cells.
4. Effects of antidepressants on cell viability
Drug effects did not produce any significant changes in cell viability in the treated cells as demonstrated by MTT analysis at week 1 of treatment (Fig. 4).
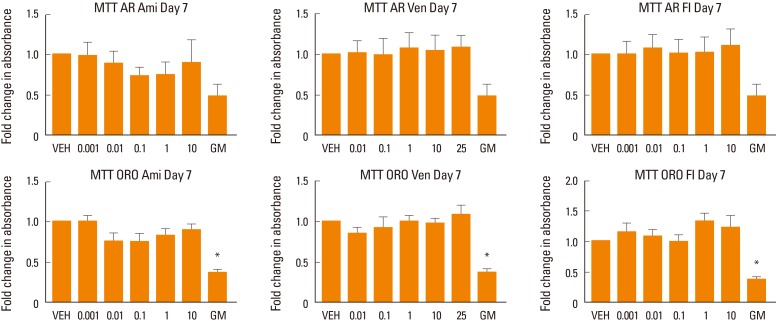
Assessment of cell viability. Human mesenchymal stem cells (MSC) induced to differentiate into osteoblasts (alizarin red [AR]) or adipocytes (oil red O [ORO]) in the presence of various concentrations of amitriptyline (Ami), venlafaxine (Ven), or fluoxetine (Fl). The Figure shows the effect of increasing concentrations of the drugs on cell viability. No significant effect was found at any treated conditions. Cells cultured in MSC growth media (GM) were used as control. *P<0.05 compared to differentiation media. VEH, vehicle.
DISCUSSION
The differentiation process of MSC in bone marrow is regulated by an intricate set of interactions between numerous molecular factors. A disruption in the balance of this mechanism ultimately results in a change in differentiation outcomes. In this study, we examined the effect of antidepressants with different mechanisms of action on differentiation of hMSC into osteoblasts and adipocytes using mineralization, alkaline phosphatase activity and lipid staining as surrogate markers for these processes. Interestingly, while fluoxetine had a strong inhibitory effect on both mineralization and adipogenesis, amitriptyline and venlafaxine had only modest effects on adipogenesis and negligible effect on mineralization. Since all three of these antidepressants have strong affinity for the 5-HTT,[232931] the effect observed here could have resulted from different levels of affinity with this molecular target, which is also associated with variable levels of serotonin.
Fluoxetine showed the greatest change in both mineralization and adipogenesis. Fluoxetine has strong affinity for serotonin receptors – particularly the 5-hydroxytryptamine (5-HT)2 receptors at the concentrations used here.[4041] Previous studies have shown that increased concentrations of serotonin can affect osteogenesis in vitro through 5-HT2 or 5-HT1 receptors and via the nuclear factor-κB or runt-related transcription factor 2 pathways,[181942] an effect that has been also reported in vivo.[43] In contrast, amitriptyline and venlafaxine have a weaker affinity for serotonin receptors and higher affinity for a variety of receptors and neurotransmitters, which could explain the divergent effect observed in this study. Overall, our experiments allowed us to conclude that the inhibitory effects of antidepressants on mineralization are dose-dependent and associated with the level of affinity of each antidepressant for 5-HTT.
A novel observation of this study is the effect of antidepressants on adipogenesis, which has been partially explored in the past. It would be expected that inhibition of osteoblastogenesis would be associated with higher adipogenesis, however this was not the case. The observation that all our tested antidepressants affected adipogenesis suggest that this effect is also associated with the serotonin-regulated pathways. However, this effect occurred under most treatment conditions thus indicating that even low levels of serotonin activity could be a strong inhibitor of adipogenesis. The direct effect of serotonin on adipogenic pathways of hMSC should be a subject of future studies.
In conclusion, the data presented here support our hypothesis that antidepressants affect differentiation of hMSC to osteoblasts and adipocytes, and that that each class of antidepressant has a varying influence on MSC differentiation, which was previously unknown, and seems to be dependent on their affinity for 5-HTT–a hypothesis that deserves further exploration. As the use of antidepressants in clinical practice has dramatically increased in recent years, understanding how these drugs are associated with osteoporosis and fracture risk is pivotal and this may influence our prescribing practices as our knowledge increases.
ACKNOWLEDGMENTS
This study was funded by grants from the Australian Institute for Musculoskeletal Science (AIMSS) and the Nepean Medical Research Foundation.
Notes
No potential conflict of interest relevant to this article was reported.