|
 |
| jbm > Volume 25(1); 2018 > Article |
|
Abstract
Sarcopenia was listed in the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) as M62.84, on October 1, 2016. Sarcopenia is primarily associated with metabolic diseases, such as diabetes, obesity, and cachexia, as well as chronic renal failure, congestive heart failure, and chronic obstructive pulmonary disease. Sarcopenia is also significantly associated with osteoporosis in elderly populations and the combined disease is defined as osteosarcopenia. Several studies have confirmed that sarcopenia and osteoporosis (osteosarcopenia) share common risk factors and biological pathways. Osteosarcopenia is associated with significant physical disability, representing a significant threat to the loss of independence in later life. However, the pathophysiology and diagnosis of osteosarcopenia are not fully defined. Additionally, pharmacologic and hormonal treatments for sarcopenia are undergoing clinical trials. This review summarizes the epidemiology, pathophysiology, diagnosis, and treatment of osteosarcopenia, and includes Korean data.
The world's population is expected to age rapidly in most regions and people are living longer. This situation is similar in Korea. Korea became an aging society (elderly population ≥7% of the total population) in 2000. In 2018, Korea will become an aged society (defined as an elderly population ≥14% of the total population), and by 2026 will be a super-aged society (elderly population ≥20% of the total population). Increased elderly populations are increasing prevalence of many chronic diseases including osteoporosis and sarcopenia.[1]
Although osteoporosis related studies on the elderly population have reported clinical outcomes and established treatment guidelines in the last decades, sarcopenia and related studies are getting popular, recently. In addition, sarcopenia have been the International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) as M62.84 since October 1, 2016.
Sarcopenia have been mostly manifested in metabolic diseases, such as diabetes, obesity, cachexia, and some specific diseases, including chronic renal failure, congestive heart failure, and chronic obstructive pulmonary disease. However, sarcopenia is significantly associated with osteoporosis in elderly populations. Several studies have confirmed that sarcopenia and osteoporosis (osteosarcopenia) share common risk factors and biological pathways and osteosarcopenia are associated with significant physical disability, representing significant threat to loss of independence in later life.[2] The combination of these two diseases exacerbates negative health outcomes and has been described as a “hazardous duet” adding the propensity of falling from sarcopenia to vulnerability of bones in those with osteoporosis.[3] The combined effect of sarcopenia and osteoporosis represents a serious problem in the elderly.
This review describes epidemiology, pathophysiology, diagnosis, and treatment of osteosarcopenia including Korean data.
Bone mineral density (BMD) has been used as a diagnostic tool to identify risk factors for osteoporosis. However, low BMD may result from reduced peak bone mass achieved during growth and/or excessive bone loss in later life. Natural course of bone loss in post-menopausal women documented that decline in BMD is linear with age at the hip with a rate of 1.67%, but quadratic at the spine with average initial loss of 3.12% occurring during middle age.[4] Prevalence of osteoporosis in 4,946 adults age 50 or older using data from the Korea National Health and Nutrition Examination Survey (KNHANES) 2008 to 2009 was 35.5% in women and 7.5% in men.[5] Recently, longitudinal follow-up study 2004 to 2015 in the same cohort was reported that prevalence of osteoporosis increased from 48.4% to 66.1% (42.7% for men and 74.4% for women).[6]
In Korea, at age 50, residual lifetime probabilities of osteoporosis-related fractures are 59.5% for women and 23.8% for men.[7] Incidence of hip fracture increased in Korean populations older than age 50 2008 to 2012, and the number of fractures was predicted to increase by 1.4 times (35,729 in 2016 to 51,259 in 2025) by 2025.[8]
Lean muscle mass contributes up to approximately 50% of total body weight in young adults, but decreases with age to approximately 25% of total body weight by age 75 to 80.[8] Declining muscle mass in lower extremities with aging most significantly impacts mobility status, and cross-sectional area of the vastus lateralis (quadriceps) muscle decreases by up to 40% age of 20 to 80.[3] Muscle strength declines by 1.5% age 50 to 60 and by 3% thereafter.[9]
Recently, prevalence of sarcopenia was 9.3% in Korean men and 0.2% in women older than age 65 using the appendicular skeletal muscle mass (ASM)/height (Ht)2 index with data from KNHANES. However, prevalence was 10.4% in men and 10.7% in women in the same study population using the ASM/weight index.[10] Using the consensus report about Asians from the Asian Working Group for Sarcopenia (AWGS), estimated prevalence of sarcopenia in elderly women was 22.1%.[11] Although prevalence of sarcopenia could vary in the same cohort due to different diagnostic criteria, prevalence of sarcopenia will be increasing due to increase in aging populations.
Osteosarcopenia is a recently developing disease entity, so its epidemiology is seldom reported. Prevalence of sarcopenia in hip fracture patients based on Japanese criteria (appendicular skeletal muscle index [SMI] <5.46 kg/m2 in women, <6.87 kg/m2 in men) was 44.7% in women and 81.1% in men.[12] Prevalence of sarcopenia in patients with hip fracture based on the New Mexico Elder Health Survey (height adjusted appendicular SMI<2 standard deviation [SD] in a young reference group) was 64% in women and 95% in men.[13] Yoo et al.[14] reported that, using the AWGS definition, prevalence of sarcopenia in women and men with hip fracture was 44.3% and 68.2%, respectively. Yoo et al.[15] conducted further studies to determine prevalence of osteosarcopenia and the relationship of osteosarcopenia and mortality in 342 patients (83 men and 259 women) with hip fracture. Prevalence of each category in men and women (normal, osteoporosis only, sarcopenia only and osteosarcopenia) were 23%, 19%, 21% and 37%, and 21%, 49%, 6%, and 24%, respectively. They reported that prevalence of each category (normal, osteoporosis only, sarcopenia only and osteosarcopenia) were 21.6%, 41.5%, 9.6%, and 27.2%, respectively. A one-year mortality of osteosarcopenia (15.1%) was higher than that of other groups (normal, 6.8%; osteoporosis only, 4.9%; sarcopenia only, 9.1%) and osteosarcopenia after adjusting for covariates had a 1.8 times higher mortality rate than non-osteosarcopenia (hazard ratio [HR], 1.84; 95% confidence interval [CI], 0.69-4.92).
Huo et al.[16] conducted a cross-sectional study using 680 community-dwelling older individuals and reported that percentage of osteosarcopenia was almost 40%. A study of community dwelling Chinese elders older than age 65 found prevalence of osteosarcopenia in 10.4% of men and 15.1% of women.[17] Although prevalence of osteosarcopenia may be different in each study group, patients with osteosarcopenia are more susceptible to occurrence of fragility fracture, poor quality of life and higher mortality.[16,17] Drey et al.[18] reported higher rate of prevalence of osteosarcopenia (28%) than sarcopenia (21%) and osteopenia (25%) alone using 68 prefrail elders age 65 to 94 (Table 1).
Bone and muscle are strongly integrated organs with shared critical functions in structure, strength, and motion. During the aging process, function of bone and muscle is compromised and imbalanced. Therefore, pathophysiology of sarcopenia and osteoporosis in the elderly are similar and reveals overlapping features.
To explain common pathophysiology of osteosarcopenia, various causal factors including cellular and tissue interconnection, genetic, and environment have been suggested. Bone and muscles have an intensive and complex interaction with mechanical loading (mechanotransduction mechanism) and biochemical signaling pathways.[19] Skeletal muscle and bone are regulated by a variety of factors that include changes in mechanical loading. The mechanical relationship between skeletal muscle and bone has been simplified to muscle contractions serving to load and bones acting as attachment sites. This physical coupling of the two tissues is most appreciated in development, as they share a common mesenchymal precursor and synchronously develop based on perceived mechanical stimuli.[20] In these communications, several osteokine and myokines are strongly related with bone and muscle. These hormonal changes result in osteosarcopenia.
Bone and muscle are similarly originated from the paraxial mesoderm during embryonic development and are influenced by similar genetic factors. Of possible genetic factors, genetic polymorphisms of angiotensin 1 converting enzyme 1, α-actinin 3 (ACTN3), myostatin, ciliary neurotrophic factor, ACTN3 polymorphism, and vitamin D receptor influence sarcopenia and osteoporosis.[21,22,23,24] Additionally, these pleiotropic regulation of genes from multiple pathways, including inflammatory, growth hormone, and steroid metabolism, leptin, transcription factor sex-determining region Y-box 17, pleiotrophin, vascular endothelial growth factor, and glucocorticoid receptor are associated with muscle and bone loss.[25] Genetic factors including various gene polymorphisms have influence on susceptibility to sarcopenia and osteoporotic status.[26,27]
Possible causal factors of osteosarcopenia are related with endocrine metabolism abnormality including diabetes, vitamin D deficiency, cachexia, obesity, and malnutrition.[28,29]
Consequently, sarcopenia may contribute to the evolution of low BMD and vice versa. The risk factors of osteosarcopenia and the relationship of muscle and bone in the development of osteosarcopenia.[28]
BMD is measured by dual energy X-ray absorptiometry (DXA). Osteoporosis was defined as a BMD 2.5 SD below the peak bone mass of a young, healthy, gender- and race-matched reference population according to the World Health Organization (WHO) diagnostic classification; Normal, T-score of total femur, femoral neck and lumbar spine at either site ≥−1; Osteopenia, −2.5< the lowest T-score <−1; Osteoporosis, the lowest T-score ≤−2.5.[23] However, BMD alone is insufficient to identify all individuals with high risk because osteoporotic fractures may occur in patients with any given T-score.[2] Thus, a number of clinical risk factors that provide information on fracture risk independent of BMD have been identified.[3,8,9,10,11,12,13,14] Recently, several algorithms including the WHO fracture-risk assessment tool (FRAX) algorithm,[16] Q fracture algorithm,[17] and Garvan Fracture Risk Calculator (Garvan) [18,19] have been developed to estimate fracture probability using additional risk factors for fracture. Recently, Korean Fracture Risk Score (KFRS) with observed risks at 7 years within each 10th of predicted risk have been available since 2016.
During the follow-up period, 19,840 osteoporotic fractures were reported (4,889 in men and 14,951 in women) in the development dataset. The assessment tool called the KFRS is comprised of a set of nine variables, including age, body mass index, recent fragility fracture, current smoking, high alcohol intake, lack of regular exercise, recent use of oral glucocorticoid, rheumatoid arthritis, and other causes of secondary osteoporosis.[30]
Sarcopenia is diagnosed with lower lean body mass, muscle strength, and gait speed. Body composition is measured by whole-body DXA or bioelectrical impedance analysis (BIA). Bone mineral content, fat mass, and lean soft tissue mass are measured separately for each part of the body, including arms and legs. Recently four of main concerns, the European Working Group on Sarcopenia in Older People (EWGSOP) definition of sarcopenia, the International Working Group on Sarcopenia (IWGS), definition of the American Foundation for the National Institutes of Health (FNIH) sarcopenia project, and, AWGS consensus are available. However, The EWGSOP, and IWGS criteria may not be applicable due to differences in ethnicity, genetic background, and body size.[31]
The AWGS consensus takes similar approaches for sarcopenia diagnosis, but unlike EWGSOP, recommends measuring muscle strength (handgrip strength) and physical performance (usual gait speed) as the screening test.[32] Another difference is cut-off values of these measurements in Asian populations that differ from those in Caucasians because of ethnicities, body size, lifestyles, and cultural backgrounds. AWGS recommends cut-off values of the appendicular SMI of 7.0 kg/m2 in men and 5.4 kg/m2 in women by using DXA and 7.0 kg/m2 in men and 5.7 kg/m2 in women, using BIA. Low handgrip strength is defined as <26 kg for men and <18 kg for women and the cut-off of 0.8 m/s for gait speed (Table 2).[32]
Osteosarcopenia is the term used to define elderly persons diagnosed with low BMD and sarcopenia. However, diagnostic adaptations of osteosarcopenia are not the same. Yoshimura et al.[33] in the Research on Osteoarthritis/Osteoporosis Against Disability (ROAD) study defined osteosarcopenia as osteoporosis (T-score≤−2.5) and the criteria defined by the AWGS. Two other studies defined osteosarcopenia as osteopenia and sarcopenia.[16,18] Yet other studies defined sarco-osteoporosis as osteoporosis and sarcopenia.[17,34] Although there was no consensus, the operational definition of the combination of osteoporosis and sarcopenia are generally accepting as osteosarcopenia or sarco-osteoporosis.[35]
Although anti-osteoporosis medications are well established in elderly populations with osteoporosis, appropriative medication for sarcopenia are under development and not available to patients with sarcopenia. Recent advances of pharmacological, hormonal, and nutritional approaches for attenuating sarcopenia have been improving. Different kinds of myostatin inhibitors and angiotensin-converting enzyme inhibitors are in clinical trials as pharmacologic treatment. Additionally, testosterone, ghrelin, and vitamin D are performing in clinical trials as humoral factors.[36]
Presently, nutrition and exercise are the best treatment methods for sarcopenia. European guidelines recommend optimal dietary protein intake of 1.0 to 1.2 g/kg body weight/day with at least 20 to 25 g of high-quality protein at each main meal and the same protein intake for healthy older people and 1.2 to 1.5 g/kg body weight/day for those malnourished or at risk of malnutrition.[37,38,39] Inadequate nutrition intake is the most significant cause of sarcopenia and osteoporosis. According to the KNHANES guidelines in 2015, 55 g/day and 45 g/day of protein or 0.91 g/kg/day are recommended for healthy elderly men and women age 65 and older. In Korea, nutritional intervention study was conducted using a protein supplement (400 kcal, 25 g protein) in a low-income elderly person age 65 or older for 12 weeks. They reported that the muscle function and frailty score improved compared to the control without intervention, respectively.[40]
In previous studies, resistance-induced strength training has been proposed as an effective intervention for sarcopenia. However, various clinical studies are currently being conducted to standardize for the types, intensity, frequency, and duration of exercise programs in patients with sarcopenia.[41] In the American College of Sports Medicine, resistance exercise recommended for elderly people is conducted 3 times a week, with an average of 30 min. In the case of an exercise program, it can be divided into 6 parts (chest, shoulder, arm, waist, abdomen, lower body) to allow complex exercises. When exercising, the larger muscle group (lower body, lower back, chest) should be conducted before the smaller muscle group (arm, shoulder, abdomen). A set of exercises should not exceed a maximum of three sets. The strength of the exercise is conducted at maximum intensity of 65% to 75% during 1 repetition maximum, and it is recommended that repetition frequency per set is 10 to 15 times based on 65% to 75%.[42] Recently, Korean guidelines for falling prevention recommended regular exercise including balance training, strengthening, aerobic, resistance exercise to prevent falling and falling risk in community dwelling elderly populations.[43]
References
1. Lim JW. The changing trends in live birth statistics in Korea, 1970 to 2010. Korean J Pediatr 2011;54:429-435.




2. Ha YC, Kim TY, Lee A, et al. Current trends and future projections of hip fracture in South Korea using nationwide claims data. Osteoporos Int 2016;27:2603-2609.



3. Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 1995;50 Spec No:11-16.

4. Zhai G, Hart DJ, Valdes AM, et al. Natural history and risk factors for bone loss in postmenopausal Caucasian women: a 15-year follow-up population-based study. Osteoporos Int 2008;19:1211-1217.



5. Choi YJ, Oh HJ, Kim DJ, et al. The prevalence of osteoporosis in Korean adults aged 50 years or older and the higher diagnosis rates in women who were beneficiaries of a national screening program: the Korea National Health and Nutrition Examination Survey 2008-2009. J Bone Miner Res 2012;27:1879-1886.


6. Kwon YJ, Park KS, Choi BH, et al. Prevalence of osteoporosis and effectiveness of screening test using ultrasound bone densitometry and education in a community-dwelling population. J Korean Med Sci 2017;32:352-356.


7. Park C, Ha YC, Jang S, et al. The incidence and residual life-time risk of osteoporosis-related fractures in Korea. J Bone Miner Metab 2011;29:744-751.



8. Short KR, Vittone JL, Bigelow ML, et al. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab 2004;286:E92-E101.


9. von Haehling S, Morley JE, Anker SD. From muscle wasting to sarcopenia and myopenia: update 2012. J Cachexia Sarcopenia Muscle 2012;3:213-217.



10. Kim KM, Jang HC, Lim S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J Intern Med 2016;31:643-650.




11. Kwon HJ, Ha YC, Park HM. Prevalence of sarcopenia in the Korean woman based on the Korean national health and nutritional examination surveys. J Bone Metab 2016;23:23-26.



12. Hida T, Ishiguro N, Shimokata H, et al. High prevalence of sarcopenia and reduced leg muscle mass in Japanese patients immediately after a hip fracture. Geriatr Gerontol Int 2013;13:413-420.


13. Di Monaco M, Castiglioni C, Vallero F, et al. Sarcopenia is more prevalent in men than in women after hip fracture: a cross-sectional study of 591 inpatients. Arch Gerontol Geriatr 2012;55:e48-e52.


14. Yoo JI, Ha YC, Kwon HB, et al. High prevalence of sarcopenia in Korean patients after hip fracture: a case-control study. J Korean Med Sci 2016;31:1479-1484.



15. Yoo JI, Kim H, Ha YC, et al. Osteosarcopenia in patients with hip fracture Is related with high mortality. J Korean Med Sci 2018;33:e27.

16. Huo YR, Suriyaarachchi P, Gomez F, et al. Phenotype of osteosarcopenia in older individuals with a history of falling. J Am Med Dir Assoc 2015;16:290-295.


17. Wang YJ, Wang Y, Zhan JK, et al. Sarco-osteoporosis: prevalence and association with frailty in Chinese community-dwelling older adults. Int J Endocrinol 2015;2015:482940




18. Drey M, Sieber CC, Bertsch T, et al. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res 2016;28:895-899.


19. Goodman CA, Hornberger TA, Robling AG. Bone and skeletal muscle: key players in mechanotransduction and potential overlapping mechanisms. Bone 2015;80:24-36.



21. Garatachea N, Lucía A. Genes and the ageing muscle: a review on genetic association studies. Age (Dordr) 2013;35:207-233.



22. Tan LJ, Liu SL, Lei SF, et al. Molecular genetic studies of gene identification for sarcopenia. Hum Genet 2012;131:1-31.



23. Cho J, Lee I, Kang H. ACTN3 gene and susceptibility to sarcopenia and osteoporotic status in older Korean adults. Biomed Res Int 2017;2017:4239648




24. González-Mercado A, Sánchez-López JY, Regla-Nava JA, et al. Association analysis of vitamin D receptor gene polymorphisms and bone mineral density in postmenopausal Mexican-Mestizo women. Genet Mol Res 2013;12:2755-2763.


25. Karasik D, Kiel DP. Genetics of the musculoskeletal system: a pleiotropic approach. J Bone Miner Res 2008;23:788-802.



26. Reed T, Fabsitz RR, Selby JV, et al. Genetic influences and grip strength norms in the NHLBI twin study males aged 59-69. Ann Hum Biol 1991;18:425-432.


27. Arden NK, Spector TD. Genetic influences on muscle strength, lean body mass, and bone mineral density: a twin study. J Bone Miner Res 1997;12:2076-2081.


28. Kang SY, Lim GE, Kim YK, et al. Association between sarcopenic obesity and metabolic syndrome in postmenopausal women: a cross-sectional study based on the Korean national health and nutritional examination surveys from 2008 to 2011. J Bone Metab 2017;24:9-14.



30. Kim HY, Jang EJ, Park B, et al. Development of a Korean fracture risk score (KFRS) for predicting osteoporotic fracture risk: Analysis of data from the Korean national health insurance service. PLoS One 2016;11:e0158918.



31. Lee WJ, Liu LK, Peng LN, et al. Comparisons of sarcopenia defined by IWGS and EWGSOP criteria among older people: results from the I-Lan longitudinal aging study. J Am Med Dir Assoc 2013;14:528.e1-528.e7.

32. Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc 2014;15:95-101.


33. Yoshimura N, Muraki S, Oka H, et al. Is osteoporosis a predictor for future sarcopenia or vice versa? Four-year observations between the second and third ROAD study surveys. Osteoporos Int 2017;28:189-199.



34. Buehring B, Krueger D, Binkley N. Effect of including historical height and radius BMD measurement on sarco-osteoporosis prevalence. J Cachexia Sarcopenia Muscle 2013;4:47-54.


35. Bruyère O, Cavalier E, Reginster JY. Vitamin D and osteosarcopenia: an update from epidemiological studies. Curr Opin Clin Nutr Metab Care 2017;20:498-503.

36. Sakuma K, Yamaguchi A. Recent advances in pharmacological, hormonal, and nutritional intervention for sarcopenia. Pflugers Arch 2018;470:449-460.



37. Kanis JA, McCloskey EV, Johansson H, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 2013;24:23-57.


38. Deutz NE, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr 2014;33:929-936.



39. Bauer J, Biolo G, Cederholm T, et al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc 2013;14:542-559.


40. Kim CO, Lee KR. Preventive effect of protein-energy supplementation on the functional decline of frail older adults with low socioeconomic status: a community-based randomized controlled study. J Gerontol A Biol Sci Med Sci 2013;68:309-316.



41. Peterson MD, Rhea MR, Sen A, et al. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev 2010;9:226-237.



Table 2
Main consensus criteria for sarcopenia
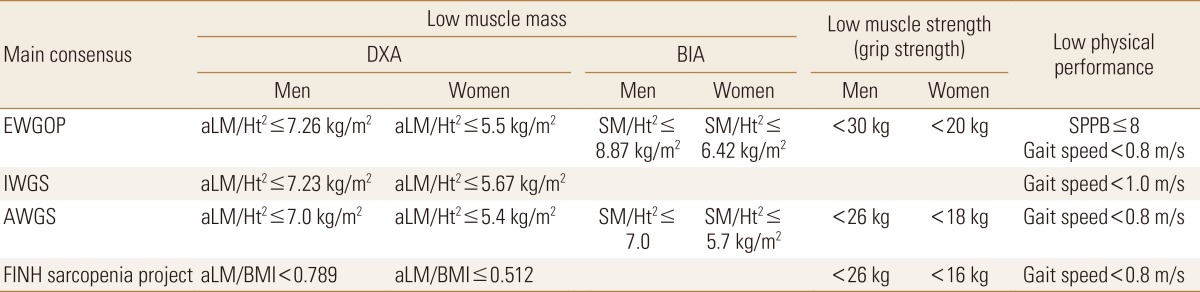
DXA, dual energy X-ray absorptiometry; BIA, bioelectrical impedance analysis; EWGSOP, European Working Group on Sarcopenia in Older People; IWGS, International Working Group on Sarcopenia; AWGS, Asian Working Group for Sarcopenia; FNIH, Foundation for the National Institutes of Health; aLM, appendicular lean mass; Ht2, height; SM, skeletal muscle; BMI, body mass index; SPPB, short physical performance battery.
- TOOLS
-
METRICS

-
- 24 Crossref
- 0 Scopus
- 3,696 View
- 57 Download
- Related articles
-
Clinician s Guide to Prevention and Treatment of Osteoporosis2008 November;15(2)
New Guidelines for the Diagnosis and Fracture Risk Assessment of Osteoporosis2008 May;15(1)




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print