|
 |
| jbm > Volume 24(1); 2017 > Article |
|
Abstract
Background
The relationship between dehydroepiandrosterone sulfate (DHEA-S) and bone mineral density (BMD) is controversial. And findings of most studies that have investigated this relationship are restricted to postmenopausal women. In this study, we investigated the relationship between serum DHEA-S and BMD in both men and women.
Methods
This cross-sectional study evaluated a total of 294 healthy Korean participants through a medical examination program. And a subgroup of 154 participants was subjected to a longitudinal analysis. We measured BMD by dual energy X-ray absorptiometry and assayed DHEA-S by a chemiluminescent immunoassay.
Results
We evaluated the association between serum DHEA-S concentration and BMD at the femur trochanter after adjusting for cofounders such as age, body mass index, lifestyle factors, serum cortisol level, serum insulin-like growth factor 1 (IGF-1) level, and sex. Through our longitudinal study, we found that the changes in BMD at the total spine, at the femur neck, and at the femur trochanter were all smaller in the ΔDHEA-S <0 group than in the ΔDHEA-S >0 group.
Osteoporosis is a common disorder characterized by compromised bone strength, an age-related bone loss, and an increased risk for fractures, affecting both men and women. This age-related degeneration begins soon after reaching peak bone mass and continues through to senescence.[1] Because the prevalence of osteoporosis and osteoporotic bone fractures increases with age and the Korea population is aging at an alarming rate, these diseases are expected to impose a heavy burden on the health system in the future. For these reasons, they are also becoming recognized as important public health concerns[2] and as serious public health threats.[3]
Osteoporosis is defined on the basis of the assessment of bone mineral density (BMD), which remains the single best predictor of primary osteoporotic fractures.[4] BMD decreases with age, and this age-related decline has been shown to be associated with factors such as race, genetics, hormones, lifestyle, and medication.
Dehydroepiandrosterone (DHEA) is the most plentiful steroid hormone secreted mainly from the adrenal gland and exists predominantly in a sulphated form (DHEA sulfate [DHEA-S]).[5] DHEA/DHEA-S production in humans decreases profoundly with age; and soon after the peak serum levels of DHEA and of DHEA-S are achieved in early adulthood, their concentration steadily declines.[6] This age-related decline in DHEA is associated with many age-related involuntary changes such as osteoporosis.[7] Several studies have reported that DHEA plays an important role in bone metabolism[8,9] and has a positive effect on BMD in both men and women.[10] However, most former studies investigating the association between DHEA-S levels and BMD have been studied principally on menopausal women and have conflicting results.[11,12] Moreover, only few longitudinal studies have investigated this subject.
We carried out a cross-sectional study to examine the association between DHEA-S levels and BMD among both healthy Korean men and women and an additional longitudinal study to evaluate the relationship between changes in serum DHEA-S levels and changes in BMD over 3 years.
We reviewed medical records of 329 consecutive adults who voluntarily participated in a physical examination at Chaum Life Center, CHA University Schooal of Medicine, Seoul, Korea, between September 2010 and June 2015. We recruited a total of 294 participants in whom BMD and serum DHEA-S concentrations had been measured and who had fulfilled the inclusion criteria of a community-dwelling healthy Korean. Among them, we selected a subgroup of 154 participants whose annual BMD measurements and blood samples for DHEA-S had been collected for four years so that we could investigate the association between change in serum DHEA-S levels (ΔDHEA-S) and change in BMD (ΔBMD). We excluded those who had cancer, uncontrolled diabetes mellitus (hemoglobin A1c [HbA1c] >8.0% / total hemoglobin [THb]), severe kidney disease (Creatinine >2.5 mg/dL or estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2), severe lung disease (FEV1<60%), severe liver disease (serum glutamate-oxaloacetate transaminase [SGOT]/serum glutamate-pyruvate transaminase [SGPT] >80 IU/L), mental disease or drug abuse and taken drugs which may affect bone metabolism. Women who were pregnant or lactating were also excluded. This study was approved by the Institutional Review Board of CHA University School of Medicine.
All physical examinations were performed by medical staff in accordance to standard procedures. Participants were asked about lifestyle behaviors (including exercise, cigarette smoking, and alcohol consumption) and any ongoing treatments for disease. If a participant was undergoing treatment at the time of enrollment, we gathered information regarding the diagnosis, the date of diagnosis, and the list of medications with which the patient has been administered. Trained staff reviewed the completed questionnaires and entered the responses into a database.
Participants were classified by smoking status as non-smokers, ex-smokers, or current smokers and by alcohol consumption as non-drinkers, abstainers (<70 g/week of alcohol), or current drinkers (≥70 g/week of alcohol). Regular exercise was defined as exercising more than twice a week. Body weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, with subjects wearing light indoor clothing without footwear. The body mass index (BMI) was calculated as the ratio of weight to height squared (kg/m2). Blood samples were obtained from the antecubital vein of the participant early in the morning after a 12-hr overnight fast. We used a chemiluminescent immunoassay for the quantitative determination of DHEA-S (UniCel DxI 800; Beckman Coulter,Fullerton, CA, USA). ΔDHEA-S and ΔBMD were calculated by (fourth-year result) − (first-year result).
Using dual energy X-ray absorptiometry (DXA), we measured BMD in 4 skeletal sites: the lumbar spine (L1-L4), the femur neck, the femur ward, and the femur trochanter (Hologic Discovery-W; Hologic Inc., Bedford, MA, USA).
All continuous and categorical variables were reported as the mean±standard deviation (SD) and as percentages. Using Pearson's correlation, we measured the strength of the correlation between DHEA-S and BMD at the various skeletal sites. Multiple linear regression analysis was used to assess these associations after adjusting for confounding variables including age, BMI, smoking, alcohol consumption, serum cortisol level, and serum insulin-like growth factor 1 (IGF-1) level. We divided the subjects into three groups according to serum DHEA-S concentrations and then evaluated the groups through one-way-analysis of variance (ANOVA). For the 3-year longitudinal study, we divided the subgroup into two according to the change in serum DHEA-S levels and used the paired t-test to analyze the statistical significance of differences between the two groups. All analyses were performed with IBM SPSS statistics version 18.0 (SPSS Inc., Chicago, IL, USA). And statistical significance was determined by a P-value <0.05.
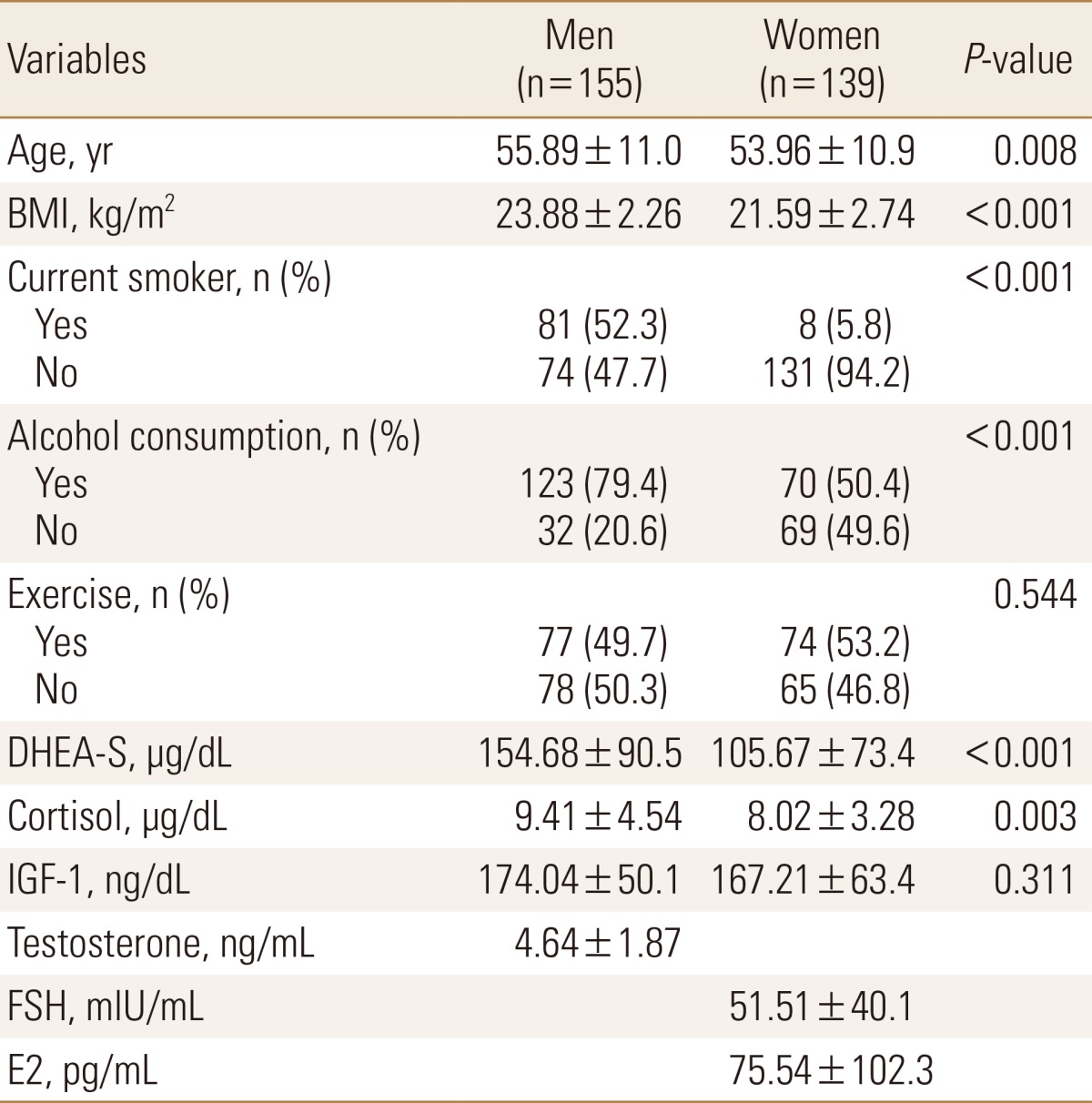
The baseline characteristics of the 294 subjects in the study are presented in Table 1. The mean age was 54.9±11.0 years (range, 16-85 years), and the mean serum DHEA-S level was 131.5±86.3 µg/dL (range, 4.8-677.7 µg/dL).
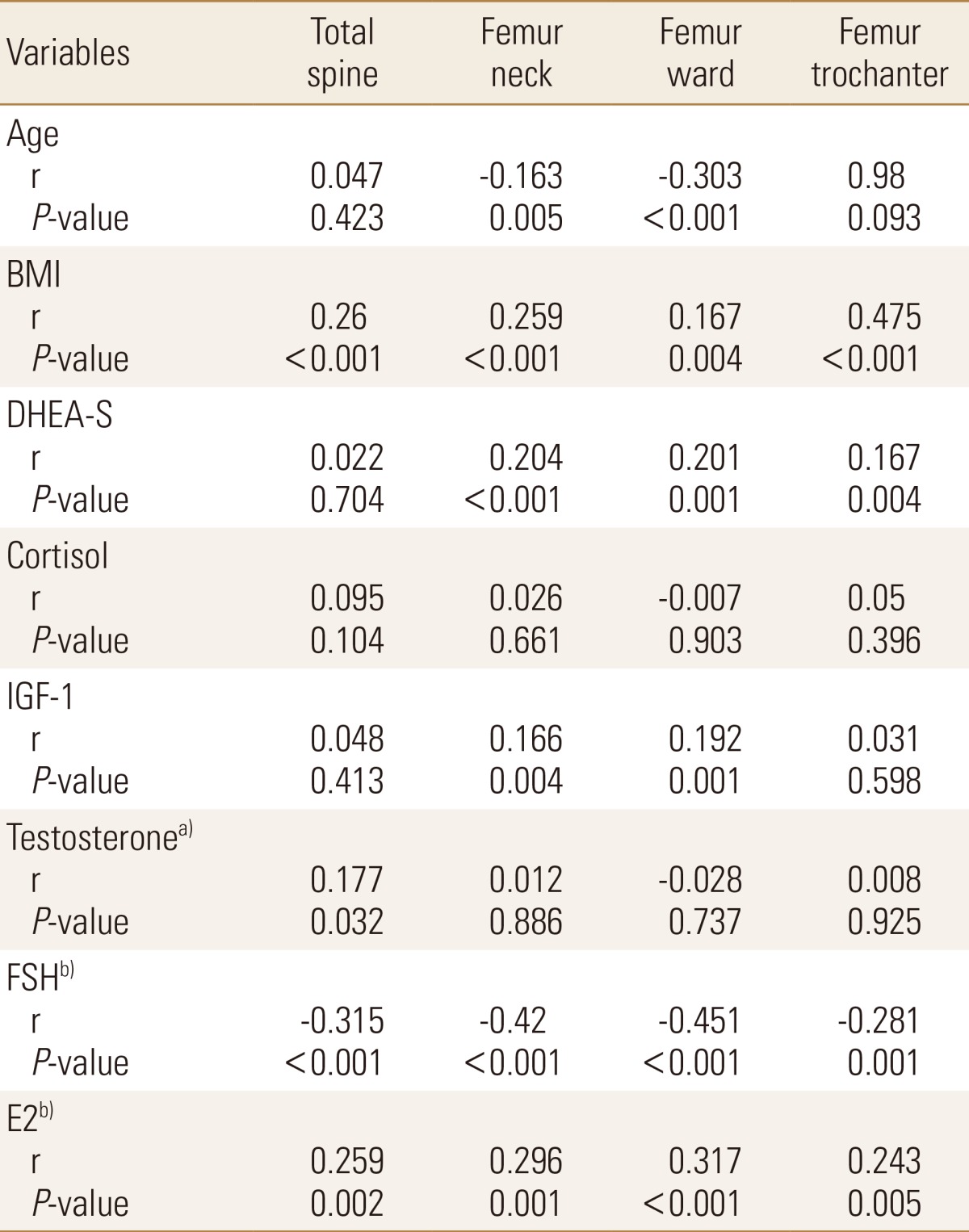
Pearson correlation coefficients of BMD with age, BMI, smoking, alcohol consumption and serum hormone levels are presented in Table 2. We found that age was positively correlated with BMI at the femur neck and at the femur ward. Conversely, BMI, DHEA-S level, and IGF-1 level were all positively correlated with BMD at various sites (BMI, all sites; DHEA-S, femur neck, ward, and trochanter; IGF-1, femur neck and ward). There was no association between serum cortisol level and BMD at any of the sites. Testosterone was positively correlated with BMD at only the total spine in men. We found that follicle stimulating hormone was negatively correlated with BMD and that estradiol was positively correlated with BMD at all sites in women.
The association between BMD and serum DHEA-S was analyzed after adjusting for potential confounders (Table 3). In model 1 where cofounders age and BMI were adjusted for, we found that serum DHEA-S level was positively associated with BMD at the femur neck and the trochanter. In model 2, we found that serum DHEA-S level was positively associated with BMD at the femur neck and the trochanter even after adjusting for other variables. But in model 3, after adjustment for sex, there was no significant association between DHEA-S and BMD at the femur neck.
The subjects were categorized into three tertile groups (T1, T2, and T3) according to serum DHEA-S concentration and compared by one-way analysis of variance (Fig. 1). We found that BMD at the femur neck, at the ward, and at the trochanter increased in a dose-dependent fashion across increasing DHEA-S tertile categories. Compared to subjects in the lowest DHEA-S tertile category (T1), those in the higher DHEA-S categories (T2, T3) showed significantly higher BMD at the femur neck, at the ward, and at the trochanter.
The subgroup (n=154) was divided into two groups (G1, n=72; G2, n=82) according to the change in serum DHEA-S levels over 3 years. As seen on Figure 2, which shows the mean change in BMD by group, we found that the change in BMD was lower in the ΔDHEA-S <0 group than in the ΔDHEA-S >0 group at the total spine, at the femur neck, and at the femur trochanter. The change in BMD did not significantly differ between the two groups at the femur ward.
We investigated the correlation between BMD and serum DHEA-S levels in healthy Koreans. Our data suggest that serum DHEA-S level is positively correlated with BMD, especially with BMD in the femur. Even after adjusting for potential confounders, such as age, BMI, sex, lifestyle, and other hormones, we found that BMD at the femur neck and at the femur trochanter was associated with serum DHEA-S. However after adjusting for sex, this association was no longer significant at the femur neck.
DHEA mediates its action via multiple signaling pathways and in this process converts into androgens and estrogens, leading to several downstream signaling cascades.[13,14] The role of DHEA in bone metabolism is likely to be complex. Not only does DHEA stimulate cell proliferation and alkaline phosphatase activity in human osteoblasts, it also stimulates osteoblast differentiation in human marrow mesenchymal stem cells.[15] In porcine osteoblast-like cells and porcine osteoblasts, DHEA has anabolic effects and promotes alkaline phosphatase activity and collagen I synthesis.[16] Further, DHEA has a role in the inhibition of interleukin 6 (IL-6) in skeletal muscle catabolism and in the stimulation of IGF-I-mediated mechanisms that underlie osteoanabolic events.[15,17]
Based on the findings of the previously mentioned reports, a number of clinical studies have investigated the association between serum DHEA-S and BMD. For example, Osmanagaoglu et al.[18] suggested that DHEA-S levels positively correlate with BMD in postmenopausal women and Bácsi et al.[12] reported that serum DHEA-S levels positively correlate with BMD at the lumbar spine. Recently, a Korean study showed that DHEA-S has favorable effects on bone mass and that a low serum DHEA-S level is a potential risk factor for osteoporosis in healthy Korean men.[19] However, some studies have reported that there is no significant association between DHEA-S levels and BMD.[11,20]
In our study, the data showed a positive correlation between serum DHEA-S and BMD at the femur but not at the total spine. Tok et al.[8], who reported similar findings as in our study, reported that serum DHEA-S level is the only independent predictor of BMD at the femoral neck. Although, it is well known that trabecular bone is more sensitive to gonadal steroids than cortical bone, research on the effects of DHEA-S on trabecular bone and on cortical bone is limited. A recent study showed that low levels of DHEA-S may be associated with weak cortical bone in patients with congenital adrenal hyperplasia,[21] which can be partially explained by the findings of our study. But further studies are required to confirm the relationship between DHEA-S and cortical bone.
We found that subjects in the lowest serum DHEA-S tertile had consistently lower BMD than those in higher serum DHEA-S tertiles in our study. This is similar to the results of a former study that was performed on healthy men.[19] In addition, although most of the former studies report a positive correlation between DHEA-S and BMD in men and in women, their investigations are based on a single-sex population.[12,19,22] Because our study included both sexes as a single sample, the findings of our study suggest that DHEA-S is an independent predictor of BMD change irrespective of gender.
Several studies support a beneficial role of DHEA-S in bone health. For instance, intervention studies have reported that DHEA replacement therapy improves hip BMD and spine BMD in the aged population.[23,24,25] In another study, it was shown that high baseline levels of serum DHEA-S is associated with reduced bone loss at the femur neck and the lumbar spine.[22] Similarly, using our subgroup analysis, we showed that BMD at the total spine, the femur neck, and the femur trochanter was more preserved in the ΔDHEA-S >0 group than in the ΔDHEA-S <0 group after 3 years. Our results imply that maintaining levels of serum DHEA-S can minimize the age-related bone loss. Further, our data suggest that follow-up serum DHEA-S levels may be a useful indicator of BMD in healthy subjects.
Our study has several limitations. Because the study subjects were volunteers for a physical evaluation at a single hospital, selection bias may have been introduced in the way participants were recruited, which means that our study population may not be a representative sample of the general Korean population. Further, the number of subjects studied, especially in the subgroup analysis, was not large enough to provide conclusive evidence. And because of subjects were small in number, we could not separate men and women in Figure 1 and 2. To raise the statistical power and wider representation of our study, more subjects are needed in a future study. Lastly we measured BMD at femur neck, ward and trochanter instead of total femur BMD which is widely used for diagnosing osteoporosis.
Our study showed that higher concentrations of serum DHEA-S are correlation with higher femur BMD, but not with spine BMD, in healthy Koreans. As such, we propose that maintaining serum DHEA-S level can slow down the age-related reduction in BMD. However, prospective studies with a larger sample number and longer follow-up periods are required.
References
1. Chang KP, Center JR, Nguyen TV, et al. Incidence of hip and other osteoporotic fractures in elderly men and women: Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res 2004;19:532-536.


2. Burch J, Rice S, Yang H, et al. Systematic review of the use of bone turnover markers for monitoring the response to osteoporosis treatment: the secondary prevention of fractures, and primary prevention of fractures in high-risk groups. Health Technol Assess 2014;18:1-180.

3. Yoon HK, Park C, Jang S, et al. Incidence and mortality following hip fracture in Korea. J Korean Med Sci 2011;26:1087-1092.



4. Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int 2005;16:581-589.


5. Lieberman S. An abbreviated account of some aspects of the biochemistry of DHEA, 1934-1995. Ann N Y Acad Sci 1995;774:1-15.


6. Orentreich N, Brind JL, Rizer RL, et al. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab 1984;59:551-555.



7. Dhatariya KK, Nair KS. Dehydroepiandrosterone: is there a role for replacement? Mayo Clin Proc 2003;78:1257-1273.

8. Tok EC, Ertunc D, Oz U, et al. The effect of circulating androgens on bone mineral density in postmenopausal women. Maturitas 2004;48:235-242.


9. Haden ST, Glowacki J, Hurwitz S, et al. Effects of age on serum dehydroepiandrosterone sulfate, IGF-I, and IL-6 levels in women. Calcif Tissue Int 2000;66:414-418.


10. Samaras N, Samaras D, Frangos E, et al. A review of age-related dehydroepiandrosterone decline and its association with well-known geriatric syndromes: is treatment beneficial? Rejuvenation Res 2013;16:285-294.



11. Lambrinoudaki I, Christodoulakos G, Aravantinos L, et al. Endogenous sex steroids and bone mineral density in healthy Greek postmenopausal women. J Bone Miner Metab 2006;24:65-71.



12. Bácsi K, Kósa JP, Borgulya G, et al. CYP3A7*1C polymorphism, serum dehydroepiandrosterone sulfate level, and bone mineral density in postmenopausal women. Calcif Tissue Int 2007;80:154-159.



13. Traish AM, Kang HP, Saad F, et al. Dehydroepiandrosterone (DHEA)-a precursor steroid or an active hormone in human physiology. J Sex Med 2011;8:2960-2982. quiz 2983.

14. Labrie F, Bélanger A, Luu-The V, et al. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids 1998;63:322-328.


15. Liang X, Glowacki J, Hahne J, et al. Dehydroepiandrosterone Stimulation of Osteoblastogenesis in Human MSCs Requires IGF-I Signaling. J Cell Biochem 2016;117:1769-1774.


16. Gordon CM, Glowacki J, LeBoff MS. DHEA and the skeleton (through the ages). Endocrine 1999;11:1-11.


17. Morales AJ, Nolan JJ, Nelson JC, et al. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab 1994;78:1360-1367.



18. Osmanagaoglu MA, Okumus B, Osmanagaoglu T, et al. The relationship between serum dehydroepiandrosterone sulfate concentration and bone mineral density, lipids, and hormone replacement therapy in premenopausal and postmenopausal women. J Womens Health (Larchmt) 2004;13:993-999.

19. Lee D, Kim H, Ahn SH, et al. The association between serum dehydroepiandrosterone Sulphate (DHEA-S) level and bone mineral density in Korean men. Clin Endocrinol (Oxf) 2015;83:173-179.


20. Sun AJ, Jing T, Heymsfield SB, et al. Relationship of leptin and sex hormones to bone mineral density in men. Acta Diabetol 2003;40(Suppl 1):S101-S105.



21. El-Maouche D, Collier S, Prasad M, et al. Cortical bone mineral density in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin Endocrinol (Oxf) 2015;82:330-337.


22. Ghebre MA, Hart DJ, Hakim AJ, et al. Association between DHEAS and bone loss in postmenopausal women: a 15-year longitudinal population-based study. Calcif Tissue Int 2011;89:295-302.



23. Jankowski CM, Gozansky WS, Schwartz RS, et al. Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: a randomized, controlled trial. J Clin Endocrinol Metab 2006;91:2986-2993.


Fig. 1
Mean bone mineral density (BMD) values according to dehydroepiandrosterone sulfate tertile categories. The mean estimated BMD values were calculated by one-way analysis of variance (ANOVA). *P<0.05: one-way ANOVA with Tukey's multiple comparison test.
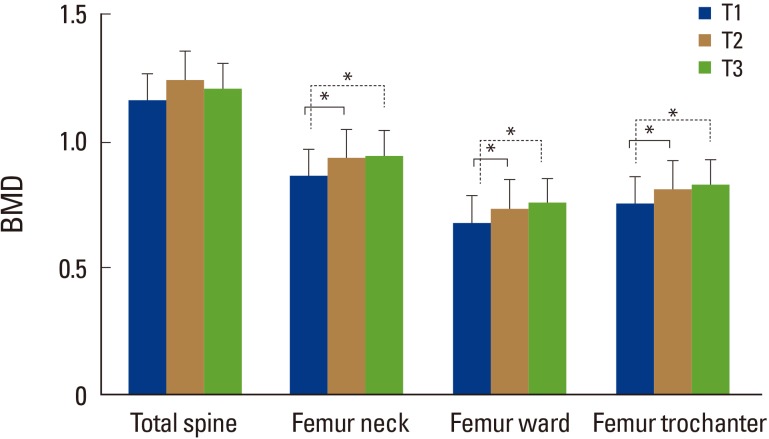
Fig. 2
Association between changes in serum dehydroepiandrosterone sulfate (DHEA-S) level and changes in bone mineral density (BMD). *P<0.05. a)ΔBMD: change in BMD. b)ΔDHEA-S: change in serum DHEA-S.

Table 3
Multiple regression analysis between bone mineral density and dehydroepiandrosterone sulfate
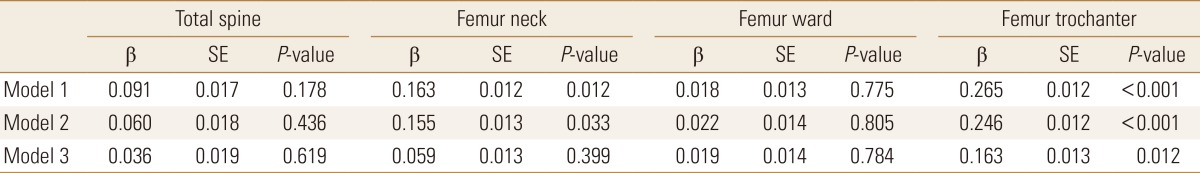
The serum dehydroepiandrosterone sulfate and cortisol levels were logarithmically transformed.
Model 1 adjusted for age and body mass index.
Model 2 additionally includes smoking, alcohol consumption, exercise, cortisol level, and Insulin-like growth factor 1 level.
Model 3 additionally includes sex.
SE, standard error.
- TOOLS
-
METRICS

-
- 11 Crossref
- 0
- 3,725 View
- 23 Download
- Related articles
-
The Association between Components of the Metabolic Syndrome and Bone Mineral Density2011 May;18(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print