Serum Sclerostin Levels in Patients with Human Immunodeficiency Virus Infection and Their Association with Bone Turnover Markers and Bone Mineral Densitometry
Article information
Abstract
Background
The aim of the study was to compare serum sclerostin levels in human im-munodeficiency virus (HIV)-infected patients and healthy controls, and to evaluate their relationship with bone turnover markers (BTM) and bone mineral density (BMD).
Methods
We prospectively studied 33 HIV treatment-naive patients and 63 healthy individuals; matched for age and sex. Serum sclerostin levels, BTM, BMD were measured. Viral load and cluster of differentiation 4 (CD4) levels were also assessed in HIV-infected patients.
Results
The mean±standard deviation (SD) age of sample was 37.6±10.3 years (range, 19 to 59 years). Of the 96 subjects, 58 (60.4%) were male and 38 (39.6%) were female. Infection with HIV is associated with significant reduction in serum sclerostin levels (HIV-infected: 39.4±28.3 vs. non HIV: 76.6±15.7 pmol/L; P<0.001) and a decrease in BMD at femoral neck and lumbar spine compared to healthy controls. Sclerostin however was not correlated with BMD and was not related to age, generally a strong correlation. There were no significant correlations between sclerostin and BTM (P>0.05).
Conclusions
These findings suggest that untreated HIV and the resulting immune deficiency and/or systemic inflammation could be an important regulator of serum sclerostin in this population.
INTRODUCTION
Patients infected with human immunodeficiency virus (HIV) can suffer fragility fractures.[12] Up to 67% of HIV-infected patients develop osteopenia and osteoporosis.[123] This has been linked to effects of antiretroviral therapy (ART), HIV virus, and the other known risk factors of osteoporosis.[345] Understanding the mechanisms of this type of osteoporosis is important to plan appropriate prevention and treatment strategies.
At a cellular level, bone remodeling is a coupled process by which mature bone tissue is removed by osteoclasts and new bone tissue is formed by osteoblasts. This process takes place continuously and is orchestrated by several signaling pathways that include molecules that interact with osteoclasts and osteoblasts, such as receptor activator of nuclear factor-kappa B ligand (RANKL) and sclerostin, respectively.[5678910] There is also a reciprocal relationship between our skeletal and immune system, which is mediated by shared molecules and cells, such as that between osteoblasts and osteoclasts, lymphocytes and osteoclasts, and osteoblasts and haematopoetic cells. For instance, rheumatoid arthritis (RA) results from abnormal/prolonged activation of immune system that eventually leads to bone destruction by bone resorbing osteoclast. [1112] In RA, the RANKL, expressed by the activated T cells (in addition to its classical expression by osteoblasts), accelerates osteoclast genesis.[12]
Sclerostin is a glycoprotein that is specifically released by osteocytes and is a potent inhibitor of the Wnt/β-catenin signaling pathway, osteoblastic differentiation, and therefore of bone formation.[678910] Sclerostin, encoded by the gene sclerosteosis (SOST), has been recognized to be a mediator of the immune system.[111213] In addition, several factors affect and interact with sclerostin in humans.[614151617] some of which might be expected to be altered in HIV population. Moreover, little information is available on the levels of circulating sclerostin among patients with HIV.
The aim of our study was to examine the changes in serum sclerostin levels among patients with HIV infection as compared with age- and sex-matched healthy seronegative HIV control group. We initially assumed that serum sclerostin levels would be higher in HIV-infected patients because low bone density is common in this population. [12345]
MATERIALS AND METHODS
1. Subjects
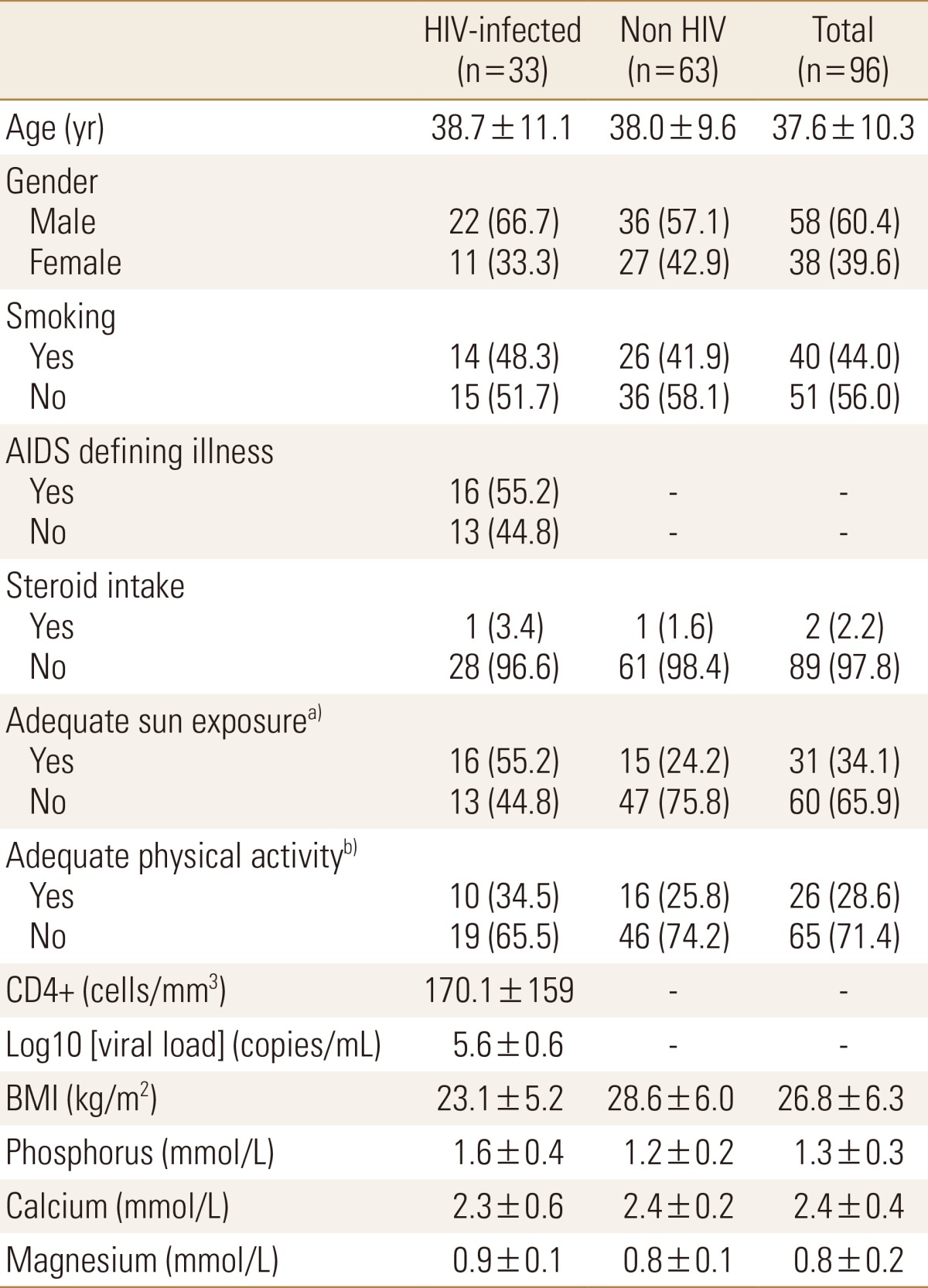
Between March 2012 and April 2013, we prospectively recruited 33 HIV-seropositive patients visiting the infectious disease clinics or admitted at King Abdulaziz University Hospital. Thirty-two were treatment-naive patients and one had begun ART for 3 months. HIV status was confirmed by Western blot. The patients were matched for age and sex with 63 HIV seronegative healthy controls chosen randomly from the community and interviewed in the Center of Excellence for Osteoporosis Research (CEOR), King Abdulaziz University. All of the participants were from Saudi Arabia. Informed consent was obtained prior to inclusion. Osteoporosis relevant data was collected from subjects using a standardized questionnaire. All of the participants underwent detailed medical examination, bone mineral density (BMD) measurement and blood and urine samples collected to measure bone turnover markers (BTM).
2. Sample collection
Venous blood samples were collected under standardized conditions, together with morning urine samples. Serum, plasma, and urine samples were stored in liquid nitrogen at -190℃ within 30 min after centrifugation at 2,500× g for 10 min.
3. Laboratory analysis
Serum sclerostin levels were measured on coded specimen and enzyme-linked immunosorbent assay (ELISA; Biomedica, Vienna, Austria). This assay uses a polyclonal goat antihuman sclerostin antibody as a capture antibody and a biotin-labeled mouse monoclonal anti-sclerostin antibody for detection. The intra- and inter-assay coefficients of variations (CVs) were 4.5% and 5.6%, respectively. Serum osteocalcin (OC) was measured using electrochemiluminescence immunoassay (ECLIA) Elecsys autoanalyzer (Roche Diagnostics GmbH, Mannheim, Germany). The intra- and inter-assay CVs were 1.8% and 1.2%, respectively. Serum cross-linked C-terminal telopeptides of type 1 collagen (CTX) was measured by Elecsys β-CrossLaps assay using ECLIA Elecsys autoanalyzer (Roche Diagnostics GmbH). The intra- and inter-assay CVs were 4%. Urinary N-telopeptide of type 1 collagen (NTX) was determined by using a competitive-inhibition ELISA (Biomedica) using Osteomark kits (Ostex International, Seattle, USA). This intra- and inter-assay CVs 7.8% and 4.5% respectively. Serum parathyroid hormone (PTH), was measured by commercially available immunoassays using a COBAS-e-411-Hitachi immunoassay autoanalyzer (Roche Diagnostics GmbH, Mannheim, Germany). Serum -25-hydroxy-vitamin D (25[OH]D) creatinine, calcium, phosphate, and magnesium were measured as described previously.[18] Cluster of differentiation 4 (CD4) counts were measured by flow cytometry (FACS Calibur; Becton Dickinson, San Jose, CA). HIV1-RNA was quantified by polymerase chain reaction (PCR) using the AMPLICOR HIV1-MONITOR Ultra-sensitive Test (version 1.5; Roche Molecular Diagnostics, Laval, Quebec, Canada) with a linear range of 100,000 to 50 copies/mL. CD4 and HIV1-RNA were measured in HIV cases only.
4. BMD measurement
BMD of the lumbar spine (LS; L1-L4) and right and left femoral neck was measured using a Hologic Discovery (Hologic, Waltham, MA, USA) for the HIV seropositive arm and a GE Lunar iDXA (GE Healthcare lunar, Madison, WI, USA) system for the healthy control arm. Manufactures standard scan and position protocols were used. The scans were analyzed using Hologic Apex and the GE Lunar EnCore software; version 13.2:5 and 13.60.033 respectively. The BMD values from both systems were converted into standardized BMD (sBMD) using the Hui et al.[19] formulas for spinal BMD and the Lu et al.[20] and Damilakis and Guglielmi [21] formulae for femur BMD to minimize the differences between the two machines. sBMD is expressed in mg/cm2 while BMD are in gm/cm2. World Health Organization (WHO) criteria was used to classify the BMD results: normal BMD with a T-score of more than -1.0 standard deviation (SD), osteopenia was defined as a T-score between -1.0 and -2.5 SD, and osteoporosis was defined as T-score equals or less than -2.5 SD.[22]
5. Ethical approval
The protocols of our study were in agreement with the ethical standards of Helsinki Declaration of 1975 as revised in the year 2008 and were approved by the Human Ethics Research Committee of CEOR.
6. Statistical analysis
Data are presented as mean±SD for continuous variables, and percentages for categorical variables. The kolmogorove-smirnov test was used to test for normal distribution. If the normality assumption was violated, the log-transformation was used to normalize the data. An independent sample t-test or Mann-Whitney's U-test were used to compare continuous data. Pearson correlation was used to investigate the association between the quantitative variables. General linear models were applied to assess the associations between selected covariates with sclerostin levels. Separate regression models adjusting for covariates were used for sclerostin subgroup analysis. Bonferroni correction was used to adjust for multiple comparisons when appropriate. A P<0.05 was considered statistically significant. Data were analyzed using the SPSS database (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Characteristics of the patients and controls
Thirty three patients (22 male, 11 female) with an average age of 38.7 years (range, 19 to 59) were enrolled in the study. The healthy controls were 63 subjects (36 male, 27 female) with an average age of 38 years (range, 19 to 59). The patients and the controls demographic characteristics are shown in Table 1 and Table 2.
2. Sclerostin in HIV-infected patients and healthy controls
Sclerostin levels were significantly lower in HIV-infected patients compared to controls (39.4±28.3 vs. 76.6±15.7 pmol/L; P<0.001) (Fig. 1). We conducted a regression analysis to investigate the influence of age and body mass index (BMI) in sclerostin levels,[23] and hence adjust for the possible influence of these variables. Serum sclerostin levels were related to BMI in both groups (P<0.001), but were not related to age. Accordingly, sclerostin levels were adjusted for BMI, and were significantly lower in HIV-infected group compared with non-HIV (F=48.9, R2-adjusted=0.45; P<0.001). The difference between sclerostin levels of patients and non-HIV group remained significant when sclerostin levels are adjusted for BMI. The results remained valid even when sclerostin levels were adjusted for both BMI and age.

This figure is a Box and Whisker's Plot that shows the mean value (middle line) of sclerostin level of groups, 75th percentile (darker shade), 25th percentile (lighter shade) and lastly the error bars of the min and max (N for human immunodeficiency virus-infected=33, N for healthy control=63). HIV, human immunodeficiency virus.
We investigated the correlation between sclerostin levels and other factors in each group using sub-group analysis. Age and sclerostin levels were not significantly correlated in HIV-infected patients (r=0.3; P=0.074), but were positively correlated in healthy controls (r=0.3; P=0.048).
3. Sclerostin levels, viral load and CD4
There was no significant correlation between sclerostin levels and CD4 (r=-0.02; P=0.909), or viral load (r=-0.1; P=0.502) in HIV-infected patients.
4. Sclerostin levels and bone markers
There was no significant association between sclerostin levels and bone markers in any group (P>0.05). There were significantly lower PTH, OC and NTX levels in HIV-infected patients compared to controls (4.8±3.6 vs. 7.3±6.0 pg/mL; P=0.032), (14.5±9.4 vs. 24.6±12.5 ng/mL; P<0.001), and (667.22±89.5 vs. 1021.6±93.4; P=0.005), respectively. CTX levels did not differ significantly between HIV-infected patients and controls (Table 2).
5. Sclerostin levels and BMD
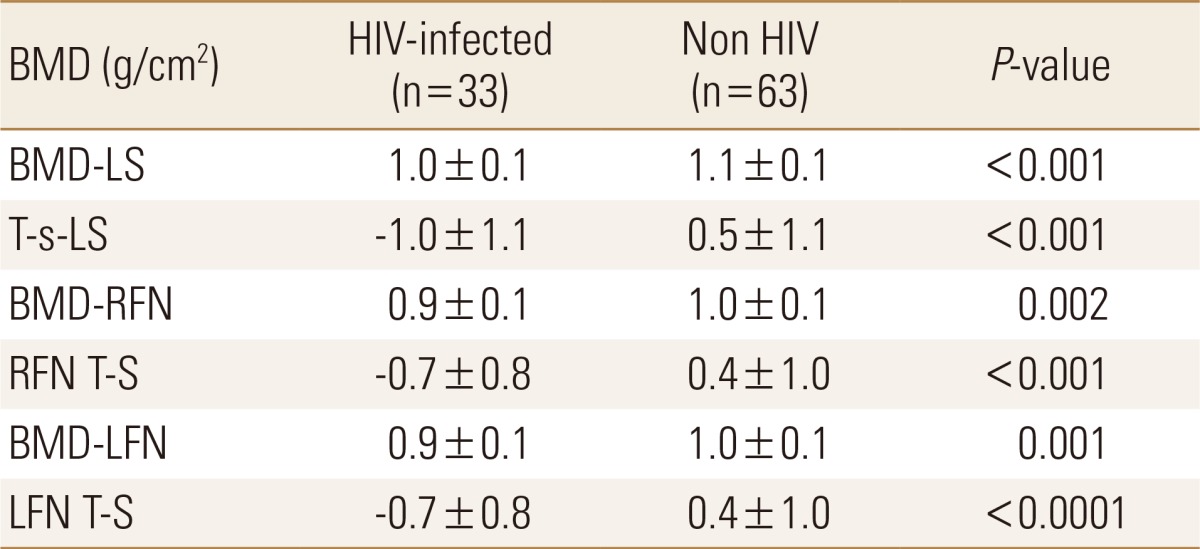
There was no significant correlation between sclerostin levels and BMD. However, BMD and T-S values at LS, right femoral neck (RFN) and left femur neck (LFN) were signifi-cantly lower in HIV-infected patients compared with controls (P<0.002) (Table 3). These results also remained significant after adjusting for BMI. However, when sBMD was used for comparison, no significant difference between groups was appreciated.
DISCUSSION
In the present study we examined the relationship between serum sclerostin levels and bone health in HIV-treatment naive patients. BMD at LS, RFN and LFN of our HIV-patients were significantly lower than controls. The effect of HIV infection on osteoblastogensis remains unclear. Sclerostin is an osteocyte-product and a potent anti-anabolic molecule; therefore when elevated, bone formation would be suppressed and hence lower BMD would result. Con-trary to what we were expecting, sclerostin levels were significantly low in HIV patients; despite their lower BMD. The BTM data (Table 2) shows a significant difference in the bone resorption marker NTX in the HIV arm compared to controls. However, OC (an anabolic-marker) is also significantly lower in the HIV-infected group, indicating a decrease in bone formation. These changes do not support the hypothesis that low serum sclerostin is a compensatory mechanism to counteract the bone resorption caused by this viral illness.[2] Patients with chronic spinal cord injury with paraplegia and low mechanical loading, are expected to have higher levels of sclerostin.[2425] However, Morse et al.[25] found that in the long-term, depleted sclerostin levels were associated with lower BMD and could represent a bio-marker of advanced disease. They that unloading in the acute-phase leads to elevated sclerostin levels which suppress osteoblastogensis[25] and with time as sclerostin producing cells decrease, along with the severity of bone loss in SCI, sclerostin levels drop making it a bio-marker for osteoporosis severity, rather than a mediator for ongoing bone loss. This could be the case with HIV, the lower sclerostin is caused by chronic decrease in sclerostin secreting osteocytes, as observed by the lower BMD. As the HIV infection progresses, the immune system deteriorates evolving to an acquired immune deficiency syndrome (AIDS) state. Infection with HIV in our community is mostly diagnosed in advanced stages, when AIDS manifestations become apparent, because screening programs are not widely available.[2627] Cain et al.[13] found that when the sclerostin producing gene is lost, B-lymphocytes growth and differentiation is affected. This suggests that sclerostin is among molecules that interact with both skeletal and immune systems with a possible negative effect of the anti-sclerostin monoclonal antibody (romosozumab) on the immune system.[12]
HIV affects both humoral and adaptive immunity, including B-lymphocytes and more profoundly T-lymphocytes, mainly the CD4+ cells.[28] Table 1 shows that 55.2% of our patients suffer from an AIDS defining illness and their mean CD4+ counts are 170 cells/mm3 indicating that our cases are in the AIDS stage of infection,[29] with a chronic, severe and debilitating status. Our cases have significant reduction in serum sclerostin levels and decrease in BMD, with no correlation for sclerostin to BMD and age (generally a strong correlation[2330]); these findings suggest that untreated HIV and the resulting immune deficiency and/or systemic inflammation may be an important regulator of serum sclerostin in this population. In a hepatitis C model of inflammation, González-Reimers et al.[31] found that sclerostin levels were slightly, but insignificantly, higher in patients compared to age and sex matched control. This finding would point more towards an immune-skeletal interaction. It may well be that a low sclerostin level simply reflects the fact that these patients are very sick with a debilitating disease and sclerostin secreting osteocytes are down-regulated as a protective measure against further general deterioration, simulating SCI to some extent.
There are a number of limitations to our study. We used two different scanners to measure BMD; however, results were adjusted using appropriate software. In addition, we did not examine patients on ART and did not test other immune parameters to trace a possible change. More information is needed to understand the role of sclerostin and its possible effect on the immune system. Another limitation in this study is the relatively small sample size. Further studies with larger sample sizes are needed to look at the relationship of sclerostin and the various stages of HIV infection, the levels of sclerostin in patients taking ART and in other immunodeficiency states and diseases.
Notes
No potential conflict of interest relevant to this article was reported.